خط زمني لاكتشافات العناصر الكيميائية
(تم التحويل من Timeline of chemical element discoveries)
The discovery of the elements known to exist today is presented here in chronological order. The elements are listed generally in the order in which each was first defined as the pure element, as the exact date of discovery of most elements cannot be accurately defined.
Given is each element's name, atomic number, year of first report, name of the discoverer, and some notes related to the discovery.
الجدول
قالب:Periodic table (discovery periods)
الاكتشافات غير المسجلة
| Z |
الاسم |
أول استعمال |
أقدم عينة متبقية |
المكتشفون | مكان أقدم عينة |
ملاحظات |
|---|---|---|---|---|---|---|
| 29 | نحاس | 9000 قبل الميلاد | 6000 قبل الميلاد | شعوب الشرق الأوسط | الأناضول | من المرجح أن يكون النحاس أول الفلزات التي اكتشفت واستعملت من قبل الإنسان.[1] تشير أبعد التقديرات إلى أن النحاس اكتشف في الشرق الأوسط وذلك حوالي 9000 سنة قبل الميلاد. يعد النحاس واحداً من أهم المواد التي تم استعمالها من قبل البشر في العصر النحاسي والعصر البرونزي. تم العثور على قلائد نحاسية تعود إلى 6000 سنة قبل الميلاد في الأناضول.[2] |
| 79 | ذهب | قبل 6000 قبل الميلاد | 5500 قبل الميلاد | شعوب الشرق الأوسط | مصر | يذهب علماء الآثار إلى الاعتقاد بأن أول استعمال للذهب بدأ مع أول نشوء للحضارات في الشرق الأوسط. ويمكن أن يكون أول فلز استعمل من قبل الإنسان. أقدم حلي ذهبي تم اكتشافه يعود للملكة المصرية زر zer.[3][4] |
| 82 | رصاص | 7000 قبل الميلاد | 3800 قبل الميلاد | في الشرق الأدنى | أبيدوس | من المعتقد أن صهر الرصاص عرف حوالي 9000 سنة قبل الميلاد، وأن أقدم المكتشفات الأثرية المصنوعة من الرصاص هو تمثال وجد في معبد أوزيريس في موقع أبيدوس الأثري ويعود إلى حوالي 3800 سنة قبل الميلاد.[5] تمت تنقية الرصاص وتمييزه عن القصدير من قبل علماء الكيمياء في عهد الدولة الإسلامية في العصور الوسطى[6] |
| 47 | فضة | قبل 5000 قبل الميلاد | ~4000 BCEقبل الميلاد | شعب الأناضول | ? | يقدر أن اكتشافه تم بعد فترة قصيرة من اكتشاف النحاس والذهب.[7][8] |
| 26 | حديد | قبل 5000 قبل الميلاد | 4000 قبل الميلاد | ? | مصر | هناك دليل على أن اكتشاف الحديد تم قبل 5000 سنة قبل الميلاد.[9] أقدم اكتشاف لأشياء حديدية استعملت من قبل الإنسان هي حلي وجدت في مصر صنعت من حديد نيزكي وذلك حوالي 4000 سنة قبل الميلاد. أدى اكتشاف الصهر حوالي 3000 سنة قبل الميلاد إلى طغيان استعمال الحديد في صناعة الأدوات والأسلحة، مما مهد لبدء العصر الحديدي قبل 1200 سنة من الميلاد.[10] |
| 6 | كربون | 3750 قبل الميلاد | ? | الفراعنة والسومريون | ? | أقدم استعمال معروف للفحم هو لاختزال خامات النحاس والزنك والقصدير لإنتاج البرونز من قبل المصريين والسومريين.[11] يعود اكتشاف الألماس غالباً إلى 2500 سنة قبل الميلاد [12] أول تحليل كيميائي مميز للكربون كان في القرن الثامن عشر الميلادي،[13] وفي عام 1789 كان الكربون مدرجاً بين العناصر من قبل أنطوان لافوازييه.[14] |
| 50 | قصدير | 3500 قبل الميلاد | 2000 قبل الميلاد | ? | ? | تم صهره مع النحاس حوالي 3500 سنة قبل الميلاد لإنتاج البرونز والنحاس الأصفر.[15] أقدم اكتشافات للقصدير تعود إلى 200 سنة قبل الميلاد.[16] تمت تنقية القصدير وتمييزه عن الرصاص من قبل علماء الكيمياء في عهد الدولة الإسلامية في العصور الوسطى (حوالي 700–1400 للميلاد).[6] |
| 16 | كبريت | قبل 2000 قبل الميلاد | ? | الصينيون والهنود | ? | أول استعمال للكبريت كان قبل حوالي 4000 سنة.[17] أول تمييز له كعنصر كان من قبل جابر بن حيان (حوالي. 800 للميلاد).[18] كما أن لافوازييه أدرجه كعنصر عام1777. |
| 80 | زئبق | قبل 2000 قبل الميلاد | 1500 قبل الميلاد | الصينيون والهنود | مصر | كان الزئبق معروفاً للصينيين القدماء والهندوس حوالي 2000 سنة قبل الميلاد، كما وجد في المقابر المصرية التي تعود إلى 1500 سنة قبل الميلاد.[19] أول تمييز له كعنصر كان من العالم جابر بن حيان (حوالي. 800 للميلاد).[18] |
| 30 | زنك | قبل 1000 قبل الميلاد | 1000 ق.م. | الهنود | شبه القارة الهندية | تم استخراج الزنك كفلز كم قبل الهنود قبل ألف سنة من الميلاد، لكن لم تفهم طبيعة هذا الفلز في تلك العصور. تم تمييزه كعنصر مستقل من قبل العالم الهندي ساموكايا حوالي 800 سنة بعد الميلاد [20] ومن قبل الخيميائي باراسيلسوس في عام 1526.[21] وعزل من قبل أندرياس زيغيزموند مارغراف عام 1746. |
الاكتشافات المسجلة
| Z | العنصر | Observed or predicted | Isolated (widely known) | Observer | First isolator | Notes |
|---|---|---|---|---|---|---|
| 15 | Phosphorus | 1669 | 1669 | H. Brand | H. Brand | Prepared from urine, it was the first element to be chemically discovered.[22] |
| 27 | Cobalt | 1732 | G. Brandt | Proved that the blue color of glass is due to a new kind of metal and not bismuth as thought previously.[23] | ||
| 78 | Platinum | 1735 | 1735 | A. de Ulloa | A. de Ulloa | First description of a metal found in South American gold was in 1557 by Julius Caesar Scaliger. Ulloa published his findings in 1748, but Sir Charles Wood also investigated the metal in 1741. First reference to it as a new metal was made by William Brownrigg in 1750.[24] |
| 28 | Nickel | 1751 | 1751 | F. Cronstedt | F. Cronstedt | Found by attempting to extract copper from the mineral known as fake copper (now known as niccolite).[25] |
| 83 | Bismuth | 1753 | C.F. Geoffroy | Definitively identified by Claude François Geoffroy in 1753.[26] | ||
| 12 | Magnesium | 1755 | 1808 | J. Black | H. Davy | Black observed that magnesia alba (MgO) was not quicklime (CaO). Davy isolated the metal electrochemically from magnesia.[27] |
| 1 | Hydrogen | 1766 | 1500 | H. Cavendish | Paracelsus | Cavendish was the first to distinguish H 2 from other gases, although Paracelsus around 1500, Robert Boyle, and Joseph Priestley had observed its production by reacting strong acids with metals. Lavoisier named it in 1793.[28][29] |
| 8 | Oxygen | 1771 | 1771 | W. Scheele | W. Scheele | Obtained it by heating mercuric oxide and nitrates in 1771, but did not publish his findings until 1777. Joseph Priestley also prepared this new air by 1774, but only Lavoisier recognized it as a true element; he named it in 1777.[30][31] |
| 7 | Nitrogen | 1772 | 1772 | D. Rutherford | D. Rutherford | He discovered Nitrogen while he was studying at the University of Edinburgh.[32] He showed that the air in which animals had breathed, even after removal of the exhaled carbon dioxide, was no longer able to burn a candle. Carl Wilhelm Scheele, Henry Cavendish, and Joseph Priestley also studied the element at about the same time, and Lavoisier named it in 1775-6.[33] |
| 17 | Chlorine | 1774 | 1774 | W. Scheele | W. Scheele | Obtained it from hydrochloric acid, but thought it was an oxide. Only in 1808 did Humphry Davy recognize it as an element.[34] |
| 25 | Manganese | 1774 | 1774 | W. Scheele | G. Gahn | Distinguished pyrolusite as the calx of a new metal. Ignatius Gottfred Kaim also discovered the new metal in 1770, as did Scheele in 1774. It was isolated by reduction of manganese dioxide with carbon.[35] |
| 56 | Barium | 1772 | 1808 | W. Scheele | H. Davy | Scheele distinguished a new earth (BaO) in pyrolusite and Davy isolated the metal by electrolysis.[36] |
| 42 | Molybdenum | 1778 | 1781 | W. Scheele | J. Hjelm | Scheele recognised the metal as a constituent of molybdena.[37] |
| 52 | Tellurium | 1782 | F.-J.M. von Reichenstein | H. Klaproth | Muller observed it as an impurity in gold ores from Transylvania.[38] | |
| 74 | Tungsten | 1781 | 1783 | T. Bergman | J. and F. Elhuyar | Bergman obtained from scheelite an oxide of a new element. The Elhuyars obtained tungstic acid from wolframite and reduced it with charcoal.[39] |
| 38 | Strontium | 1787 | 1808 | W. Cruikshank | H. Davy | Cruikshank and Adair Crawford in 1790 concluded that strontianite contained a new earth. It was eventually isolated electrochemically in 1808 by Humphry Davy.[40] |
| 1789 | A. Lavoisier | The first modern list of chemical elements – containing, among others, 29 elements of those known then.[41] He also redefined the term "element". Until then, no metals except mercury were considered elements. | ||||
| 40 | Zirconium | 1789 | 1824 | H. Klaproth | J. Berzelius | Klaproth identified a new element in zirconia.[42][43] |
| 92 | Uranium | 1789 | 1841 | H. Klaproth | E.-M. Péligot | Mistakenly identified a uranium oxide obtained from pitchblende as the element itself and named it after the recently discovered planet Uranus.[44][45] |
| 22 | Titanium | 1791 | 1825 | W. Gregor | J. Berzelius | Gregor found an oxide of a new metal in ilmenite; Martin Heinrich Klaproth independently discovered the element in rutile in 1795 and named it. The pure metallic form was only obtained in 1910 by Matthew A. Hunter.[46][47] |
| 39 | Yttrium | 1794 | 1840 | J. Gadolin | G. Mosander | Discovered in gadolinite, but Mosander showed later that its ore, yttria, contained more elements.[48][49] |
| 24 | Chromium | 1797 | 1798 | N. Vauquelin | N. Vauquelin | Vauquelin discovered the trioxide in crocoite ore, and later isolated the metal by heating the oxide in a charcoal oven.[50] |
| 4 | Beryllium | 1798 | 1828 | N. Vauquelin | F. Wöhler and A. Bussy | Vauquelin discovered the oxide in beryl and emerald, and Klaproth suggested the present name around 1808.[51] |
| 23 | Vanadium | 1801 | 1830 | M. del Río | N.G.Sefström | Río found the metal in vanadinite but retracted the claim after Hippolyte Victor Collet-Descotils disputed it. Sefström isolated and named it, and later it was shown that Río had been right in the first place.[52] |
| 41 | Niobium | 1801 | 1864 | C. Hatchett | W. Blomstrand | Hatchett found the element in columbite ore and named it columbium. Heinrich Rose proved in 1844 that the element is distinct from tantalum, and renamed it niobium which was officially accepted in 1949.[53] |
| 73 | Tantalum | 1802 | G. Ekeberg | Ekeberg found another element in minerals similar to columbite and in 1844, Heinrich Rose proved that it was distinct from niobium.[54] | ||
| 46 | Palladium | 1803 | 1803 | H. Wollaston | H. Wollaston | Wollaston discovered it in samples of platinum from South America, but did not publish his results immediately. He had intended to name it after the newly discovered asteroid, Ceres, but by the time he published his results in 1804, cerium had taken that name. Wollaston named it after the more recently discovered asteroid Pallas.[55] |
| 58 | Cerium | 1803 | 1839 | H. Klaproth, J. Berzelius, and W. Hisinger | G. Mosander | Berzelius and Hisinger discovered the element in ceria and named it after the newly discovered asteroid (then considered a planet), Ceres. Klaproth discovered it simultaneously and independently in some tantalum samples. Mosander proved later that the samples of all three researchers had at least another element in them, lanthanum.[56] |
| 76 | Osmium | 1803 | 1803 | S. Tennant | S. Tennant | Tennant had been working on samples of South American platinum in parallel with Wollaston and discovered two new elements, which he named osmium and iridium.[57] |
| 77 | Iridium | 1803 | 1803 | S. Tennant | S. Tennant | Tennant had been working on samples of South American platinum in parallel with Wollaston and discovered two new elements, which he named osmium and iridium, and published the iridium results in 1804.[58] |
| 45 | Rhodium | 1804 | 1804 | H. Wollaston | H. Wollaston | Wollaston discovered and isolated it from crude platinum samples from South America.[59] |
| 19 | Potassium | 1807 | 1807 | H. Davy | H. Davy | Davy discovered it by using electrolysis on potash.[60] |
| 11 | Sodium | 1807 | 1807 | H. Davy | H. Davy | Davy discovered it a few days after potassium, by using electrolysis on sodium hydroxide.[61] |
| 20 | Calcium | 1808 | 1808 | H. Davy | H. Davy | Davy discovered the metal by electrolysis of quicklime.[61] |
| 5 | Boron | 1808 | 1808 | L. Gay-Lussac and L.J. Thénard | H. Davy | On June 21, 1808, Lussac and Thénard announced a new element in sedative salt, Davy announced the isolation of a new substance from boracic acid soon afterwards.[62] |
| 9 | Fluorine | 1810 | 1886 | A.-M. Ampère | H. Moissan | André-Marie Ampère predicted an element analogous to chlorine obtainable from hydrofluoric acid, and between 1812 and 1886 many researchers tried to obtain this element. It was eventually isolated by Moissan.[63] |
| 53 | Iodine | 1811 | 1811 | B. Courtois | B. Courtois | Courtois discovered it in the ashes of seaweed.[64] |
| 3 | Lithium | 1817 | 1821 | A. Arfwedson | W. T. Brande | Arfwedson discovered the alkali in petalite.[65] |
| 48 | Cadmium | 1817 | 1817 | S. L Hermann, F. Stromeyer, and J.C.H. Roloff | S. L Hermann, F. Stromeyer, and J.C.H. Roloff | All three found an unknown metal in a sample of zinc oxide from Silesia, but the name that Stromeyer gave became the accepted one.[66] |
| 34 | Selenium | 1817 | 1817 | J. Berzelius and G. Gahn | J. Berzelius and G. Gahn | While working with lead they discovered a substance that they thought was tellurium, but realized after more investigation that it is different.[67] |
| 14 | Silicon | 1824 | 1824 | J. Berzelius | J. Berzelius | Humphry Davy thought in 1800 that silica was an element, not a compound, and in 1808 suggested the present name. In 1811 Louis-Joseph Gay-Lussac and Louis-Jacques Thénard probably prepared impure silicon, but Berzelius is credited with the discovery for obtaining the pure element in 1824.[68] |
| 13 | Aluminium | 1825 | 1825 | H.C.Ørsted | H.C.Ørsted | Antoine Lavoisier predicted in 1787 that alumine is the oxide of an undiscovered element, and in 1808 Humphry Davy tried to decompose it. Although he failed, he suggested the present name. Hans Christian Ørsted was the first to isolate metallic aluminium in 1825.[69] |
| 35 | Bromine | 1825 | 1825 | J. Balard and L. Gmelin | J. Balard and L. Gmelin | They both discovered the element in the autumn of 1825 and published the results the next year.[70] |
| 90 | Thorium | 1829 | J. Berzelius | Berzelius obtained the oxide of a new earth in thorite.[71] | ||
| 57 | Lanthanum | 1838 | G. Mosander | Mosander found a new element in samples of ceria and published his results in 1842, but later he showed that this lanthana contained four more elements.[72] | ||
| 68 | Erbium | 1842 | G. Mosander | Mosander managed to split the old yttria into yttria proper and erbia, and later terbia too.[73] | ||
| 65 | Terbium | 1842 | 1842 | G. Mosander | G. Mosander | In 1842 Mosander split yttria into two more earths, erbia and terbia[74] |
| 44 | Ruthenium | 1844 | 1844 | K. Claus | K. Claus | Gottfried Wilhelm Osann thought that he found three new metals in Russian platinum samples, and in 1844 Karl Karlovich Klaus confirmed that there was a new element.[75] |
| 55 | Caesium | 1860 | 1882 | R. Bunsen and R. Kirchhoff | C. Setterberg | Bunsen and Kirchhoff were the first to suggest finding new elements by spectrum analysis. They discovered caesium by its two blue emission lines in a sample of Dürkheim mineral water.[76] The pure metal was eventually isolated in 1882 by Setterberg.[77] |
| 37 | Rubidium | 1861 | R. Bunsen and G. R. Kirchhoff | R. Bunsen | Bunsen and Kirchhoff discovered it just a few months after caesium, by observing new spectral lines in the mineral lepidolite. Bunsen never obtained a pure sample of the metal, which was later obtained by Hervesy.[78] | |
| 81 | Thallium | 1861 | 1862 | W. Crookes | C.-A. Lamy | Shortly after the discovery of rubidium, Crookes found a new green line in a selenium sample; later that year, Lamy found the element to be metallic.[79] |
| 49 | Indium | 1863 | 1867 | F. Reich and T. Richter | T. Richter | Reich and Richter First identified it in sphalerite by its bright indigo-blue spectroscopic emission line. Richter isolated the metal several years later.[80] |
| 2 | Helium | 1868 | 1895 | P. Janssen and N. Lockyer | W. Ramsay, T. Cleve, and N. Langlet | Janssen and Lockyer observed independently a yellow line in the solar spectrum that did not match any other element.
Years later, Ramsay, Cleve, and Langlet observed independently the element trapped in cleveite about the same time.[81] |
| 1869 | D. I. Mendeleev | Mendeleev arranges the 64 elements known at that time into the first modern periodic table and correctly predicts several others. | ||||
| 31 | Gallium | 1875 | P. E. L. de Boisbaudran | P. E. L. de Boisbaudran | Boisbaudran observed on a pyrenea blende sample some emission lines corresponding to the eka-aluminium that was predicted by Mendeleev in 1871 and subsequently isolated the element by electrolysis.[82] | |
| 70 | Ytterbium | 1878 | 1907 | J.C.G. de Marignac | G. Urbain | On October 22, 1878, Marignac reported splitting terbia into two new earths, terbia proper and ytterbia.[83] |
| 67 | Holmium | 1878 | M. Delafontaine | Delafontaine found it in samarskite and next year, Per Teodor Cleve split Marignac's erbia into erbia proper and two new elements, thulium and holmium.[84] | ||
| 69 | Thulium | 1879 | 1879 | T. Cleve | T. Cleve | Cleve split Marignac's erbia into erbia proper and two new elements, thulium and holmium.[85] |
| 21 | Scandium | 1879 | 1879 | F. Nilson | F. Nilson | Nilson split Marignac's ytterbia into pure ytterbia and a new element that matched 1871 Mendeleev's predicted eka-boron.[86] |
| 62 | Samarium | 1879 | 1879 | P.E.L. de Boisbaudran | P.E.L. de Boisbaudran | Boisbaudran noted a new earth in samarskite and named it samaria after the mineral.[87] |
| 64 | Gadolinium | 1880 | 1886 | J. C. G. de Marignac | F. L. de Boisbaudran | Marignac initially observed the new earth in terbia, and later Boisbaudran obtained a pure sample from samarskite.[88] |
| 59 | Praseodymium | 1885 | A. von Welsbach | Von Welsbach discovered two new distinct elements in ceria: praseodymium and neodymium.[89] | ||
| 60 | Neodymium | 1885 | A. von Welsbach | Von Welsbach discovered two new distinct elements in ceria: praseodymium and neodymium.[90] | ||
| 66 | Dysprosium | 1886 | P.E.L. de Boisbaudran | De Boisbaudran found a new earth in erbia.[90] | ||
| 32 | Germanium | 1886 | A. Winkler | In February 1886 Winkler found in argyrodite the eka-silicon that Mendeleev had predicted in 1871.[91] | ||
| 18 | Argon | 1894 | 1894 | Lord Rayleigh and W. Ramsay | Lord Rayleigh and W. Ramsay | They discovered the gas by comparing the molecular weights of nitrogen prepared by liquefaction from air and nitrogen prepared by chemical means. It is the first noble gas to be isolated.[92] |
| 36 | Krypton | 1898 | 1898 | W. Ramsay and W. Travers | W. Ramsay and W. Travers | On May 30, 1898, Ramsay separated a noble gas from liquid argon by difference in boiling point.[93] |
| 10 | Neon | 1898 | 1898 | W. Ramsay and W. Travers | W. Ramsay and W. Travers | In June 1898 Ramsay separated a new noble gas from liquid argon by difference in boiling point.[93] |
| 54 | Xenon | 1898 | 1898 | W. Ramsay and W. Travers | W. Ramsay and W. Travers | On July 12, 1898 Ramsay separated a third noble gas within three weeks, from liquid argon by difference in boiling point.[94] |
| 84 | Polonium | 1898 | 1902 | P. and M. Curie | W. Marckwald | In an experiment done on July 13, 1898, the Curies noted an increased radioactivity in the uranium obtained from pitchblende, which they ascribed to an unknown element.[95] |
| 88 | Radium | 1898 | 1902 | P. and M. Curie | M. Curie | The Curies reported on December 26, 1898, a new element different from polonium, which Marie later isolated from uraninite.[96] |
| 86 | Radon | 1898 | 1910 | E. Dorn | W. Ramsay and R. Whytlaw-Gray | Dorn discovered a radioactive gas resulting from the radioactive decay of radium, isolated later by Ramsay and Gray.[97][98] |
| 89 | Actinium | 1899 | 1899 | A.-L. Debierne | A.-L. Debierne | Debierne obtained from pitchblende a substance that had properties similar to those of thorium.[99] |
| 63 | Europium | 1896 | 1901 | E.-A. Demarçay | E.-A. Demarçay | Demarçay found spectral lines of a new element in Lecoq's samarium, and separated this element several years later.[100] |
| 71 | Lutetium | 1906 | 1906 | G. Urbain and C.A. von Welsbach | G. Urbain and C.A. von Welsbach | Urbain and von Welsbach proved independently that the old ytterbium also contained a new element.[101] |
| 75 | Rhenium | 1908 | 1925 | M. Ogawa | M. Ogawa | Ogawa found it in thorianite but assigned it as element 43 instead of 75 and named it nipponium.[102] In 1922 Walter Noddack, Ida Eva Tacke and Otto Berg announced its separation from gadolinite and gave it the present name.[59] |
| 72 | Hafnium | 1911 | 1922 | G. Urbain and V. Vernadsky | D. Coster and G. von Hevesy | Urbain claimed to have found the element in rare-earth residues, while Vernadsky independently found it in orthite. Neither claim was confirmed due to الحرب العالمية الأولى. After the war, Coster and Hevesy found it by X-ray spectroscopic analysis in Norwegian zircon.[103] Hafnium was the last stable element to be discovered.[104] |
| 91 | Protactinium | 1913 | O.H.Göhring and K. Fajans | The two obtained the first isotope of this element that had been predicted by Mendeleev in 1871 as a member of the natural decay of 238U.[105] Originally isolated in 1900 by William Crookes.[106] | ||
| 43 | Technetium | 1937 | 1937 | C. Perrier and E. Segrè | C. Perrier & E.Segrè | The two discovered a new element in a molybdenum sample that was used in a cyclotron, the first synthetic element to be discovered. It had been predicted by Mendeleev in 1871 as eka-manganese.[107][108] |
| 87 | Francium | 1939 | M. Perey | Perey discovered it as a decay product of 227Ac.[109] Francium is the last element to be discovered in nature, rather than synthesized in the lab, although some of the "synthetic" elements that were discovered later (plutonium, neptunium, astatine) were eventually found in trace amounts in nature as well.[110] | ||
| 85 | Astatine | 1940 | R. Corson, R. MacKenzie and E. Segrè | Obtained by bombarding bismuth with alpha particles.[111] Later determined to occur naturally in minuscule quantities (<25 grams in earth's crust).[112] | ||
| 93 | Neptunium | 1940 | E.M. McMillan and H. Abelson | Obtained by irradiating uranium with neutrons, it is the first transuranium element discovered.[113] | ||
| 94 | Plutonium | 1940–1941 | Glenn T. Seaborg, Arthur C. Wahl, W. Kennedy and E.M. McMillan | Prepared by bombardment of uranium with deuterons.[114] | ||
| 95 | Americium | 1944 | G. T. Seaborg, A. James, O. Morgan and A. Ghiorso | Prepared by irradiating plutonium with neutrons during the Manhattan Project.[115] | ||
| 96 | Curium | 1944 | G. T. Seaborg, R. A. James and A. Ghiorso | Prepared by bombarding plutonium with alpha particles during the Manhattan Project[116] | ||
| 61 | Promethium | 1942 | 1945 | S. Wu, E.G. Segrè and A. Bethe | Charles D. Coryell, Jacob A. Marinsky, Lawrence E. Glendenin, and Harold G. Richter | It was probably first prepared in 1942 by bombarding neodymium and praseodymium with neutrons, but separation of the element could not be carried out. Isolation was performed under the Manhattan Project in 1945.[89] |
| 97 | Berkelium | 1949 | G. Thompson, A. Ghiorso and G. T. Seaborg (University of California, Berkeley) | Created by bombardment of americium with alpha particles.[117] | ||
| 98 | Californium | 1950 | S. G. Thompson, K. Street,Jr., A. Ghiorso and G. T. Seaborg (University of California, Berkeley) | Bombardment of curium with alpha particles.[118] | ||
| 99 | Einsteinium | 1952 | 1952 | A. Ghiorso et al. (Argonne Laboratory, Los Alamos Laboratory and University of California, Berkeley) | Formed in the first thermonuclear explosion in November 1952, by irradiation of uranium with neutrons; kept secret for several years.[119] | |
| 100 | Fermium | 1952 | A. Ghiorso et al. (Argonne Laboratory, Los Alamos Laboratory and University of California, Berkeley) | Formed in the first thermonuclear explosion in November 1952, by irradiation of uranium with neutrons; kept secret for several years.[120] | ||
| 101 | Mendelevium | 1955 | A. Ghiorso, G. Harvey, R. Choppin, S. G. Thompson and G. T. Seaborg | Prepared by bombardment of einsteinium with helium.[121] | ||
| 102 | Nobelium | 1958 | A. Ghiorso, T. Sikkeland, R. Walton and G. T. Seaborg | First prepared by bombardment of curium with carbon atoms.[122] | ||
| 103 | Lawrencium | 1961 | A. Ghiorso, T. Sikkeland, E. Larsh and M. Latimer | First prepared by bombardment of californium with boron atoms.[123] | ||
| 104 | Rutherfordium | 1968 | A. Ghiorso, M. Nurmia, J. Harris, K. Eskola and P. Eskola | Prepared by bombardment of californium with carbon atoms.[124] | ||
| 105 | Dubnium | 1970 | A. Ghiorso, M. Nurmia, K. Eskola, J. Harris and P. Eskola | Prepared by bombardment of californium with nitrogen atoms.[125] | ||
| 106 | Seaborgium | 1974 | A. Ghiorso, J. Nitschke, J. Alonso, C. Alonso, M. Nurmia, G. T. Seaborg, K. Hulet and W. Lougheed | Prepared by collisions of californium-249 with oxygen atoms.[126] | ||
| 107 | Bohrium | 1981 | G.Münzenberg et al. (GSI in Darmstadt) | Obtained by bombarding bismuth with chromium.[127] | ||
| 109 | Meitnerium | 1982 | G. Münzenberg, P. Armbruster et al. (GSI in Darmstadt) | Prepared by bombardment of bismuth with iron atoms.[128] | ||
| 108 | Hassium | 1984 | G. Münzenberg, P. Armbruster et al. (GSI in Darmstadt) | Prepared by bombardment of lead with iron atoms[129] | ||
| 110 | Darmstadtium | 1994 | S. Hofmann et al. (GSI in Darmstadt) | Prepared by bombardment of lead with nickel.[130] | ||
| 111 | Roentgenium | 1994 | S. Hofmann et al. (GSI in Darmstadt) | Prepared by bombardment of bismuth with nickel.[131] | ||
| 112 | Copernicium | 1996 | S. Hofmann et al. (GSI in Darmstadt) | Prepared by bombardment of lead with zinc.[132][133] | ||
| 114 | Flerovium | 1999 | Y. Oganessian et al. (JINR in Dubna) | Prepared by bombardment of plutonium with calcium[134] | ||
| 116 | Livermorium | 2000 | Y.Oganessian et al. (JINR in Dubna) | Prepared by bombardment of curium with calcium[135] |
اكتشافات غير مؤكدة
| Z | Name | Discovery year |
Discoverer | Notes |
|---|---|---|---|---|
| 118 | Ununoctium | 2002 | Joint Institute for Nuclear Research in Dubna and Lawrence Livermore National Laboratory | Prepared by bombardment of californium with calcium[136] |
| 113 | Ununtrium | 2003 | Joint Institute for Nuclear Research in Dubna and Lawrence Livermore National Laboratory | Alpha decay of ununpentium[137] |
| 115 | Ununpentium | 2003 | Joint Institute for Nuclear Research in Dubna and Lawrence Livermore National Laboratory | Prepared by bombardment of americium with calcium[137] |
| 117 | Ununseptium | 2010 | Joint Institute for Nuclear Research in Dubna and Lawrence Livermore National Laboratory | Prepared by bombardment of berkelium with calcium[138] |
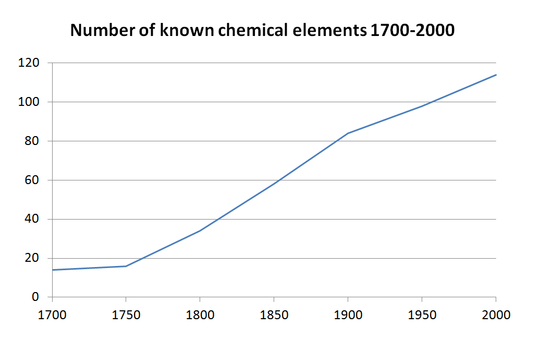
بيانيات
انظر أيضاً
الهامش
- ^ "Copper History". Rameria.com. Retrieved 2008-09-12.
- ^ CSA - Discovery Guides, A Brief History of Copper
- ^ "Gold History". Bullion.nwtmint.com. Retrieved 2008-09-12.
- ^ "The Turquoise Story". Indianvillage.com. Retrieved 2008-09-12.
- ^ "The History of Lead - Part 3". Lead.org.au. Retrieved 2008-09-12.
- ^ أ ب El-Eswed, Bassam I. (2002), "Lead and Tin in Arabic Alchemy", Arabic Sciences and Philosophy (Cambridge University Press) 12: 139–53, doi:
- ^ 47 Silver
- ^ "Silver Facts - Periodic Table of the Elements". Chemistry.about.com. Retrieved 2008-09-12.
- ^ "26 Iron". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "Notes on the Significance of the First Persian Empire in World History". Courses.wcupa.edu. Retrieved 2008-09-12.
- ^ "History of Carbon and Carbon Materials - Center for Applied Energy Research - University of Kentucky". Caer.uky.edu. Retrieved 2008-09-12.
- ^ "Chinese made first use of diamond". BBC News. 17 May 2005. Retrieved 2007-03-21.
- ^ Ferchault de Réaumur, R-A (1722). L'art de convertir le fer forgé en acier, et l'art d'adoucir le fer fondu, ou de faire des ouvrages de fer fondu aussi finis que le fer forgé (English translation from 1956). Paris, Chicago.
{{cite book}}: More than one of|author=and|last=specified (help) - ^ Senese, Fred (September 9, 2009). Who discovered carbon?. Frostburg State University. Retrieved on 2007-11-24.
- ^ "50 Tin". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "History of Metals". Neon.mems.cmu.edu. Retrieved 2008-09-12.
- ^ "Sulfur History". Georgiagulfsulfur.com. Retrieved 2008-09-12.
- ^ أ ب Strathern, Paul. (2000). Mendeleyev’s Dream – the Quest for the Elements. New York: Berkley Books.
- ^ "Mercury and the environment — Basic facts". Environment Canada, Federal Government of Canada. 2004. Retrieved 2008-03-27.
{{cite web}}: Italic or bold markup not allowed in:|publisher=(help) - ^ Craddock, P. T. et al. (1983), "Zinc production in medieval India", World Archaeology 15 (2), Industrial Archaeology, p. 13
- ^ "30 Zinc". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "15 Phosphorus". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "27 Cobalt". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "78 Platinum". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "28 Nickel". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "Bismuth". Los Alamos National Laboratory. Retrieved 3 March 2013.
- ^ "12 Magnesium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "01 Hydrogen". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ Andrews, A. C. (1968). "Oxygen". In Clifford A. Hampel (ed.). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. p. 272. LCCN 68-29938.
- ^ "08 Oxygen". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ Cook, Gerhard A.; Lauer, Carol M. (1968). "Oxygen". In Clifford A. Hampel (ed.). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 499–500. LCCN 68-29938.
- ^ Roza, Greg (2010). The Nitrogen Elements: Nitrogen, Phosphorus, Arsenic, Antimony, Bismuth. p. 7. ISBN 9781435853355.
- ^ "07 Nitrogen". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "17 Chlorine". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "25 Manganese". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "56 Barium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "42 Molybdenum". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "52 Tellurium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ IUPAC. "74 Tungsten". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "38 Strontium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "Lavoisier". Homepage.mac.com. Retrieved 2008-09-12.[dead link]
- ^ "Chronology – Elementymology". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ Lide, David R., ed. (2007–2008). "CRC Handbook of Chemistry and Physics". 4. New York: CRC Press: 42. 978-0-8493-0488-0.
{{cite journal}}:|contribution=ignored (help); Cite journal requires|journal=(help) - ^ M. H. Klaproth (1789). "Chemische Untersuchung des Uranits, einer neuentdeckten metallischen Substanz". Chemische Annalen. 2: 387–403.
- ^ E.-M. Péligot (1842). "Recherches Sur L'Uranium". Annales de chimie et de physique. 5 (5): 5–47.
- ^ "Titanium". Los Alamos National Laboratory. 2004. Retrieved 2006-12-29.
- ^ Barksdale, Jelks (1968). The Encyclopedia of the Chemical Elements. Skokie, Illinois: Reinhold Book Corporation. pp. 732–38 "Titanium". LCCCN 68-29938.
- ^ Browning, Philip Embury (1917). "Introduction to the Rarer Elements". Kongl. Vet. Acad. Handl. XV: 137.
- ^ Crell Anal. I: 313. 1796.
{{cite journal}}: Missing or empty|title=(help) - ^ Vauquelin, Louis Nicolas (1798). "Memoir on a New Metallic Acid which exists in the Red Lead of Sibiria". Journal of Natural Philosophy, Chemistry, and the Art. 3: 146.
- ^ "04 Beryllium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "23 Vanadium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "41 Niobium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "73 Tantalum". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "46 Palladium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "58 Cerium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "76 Osmium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "77 Iridium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ أ ب "45 Rhodium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "19 Potassium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ أ ب "11 Sodium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "05 Boron". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "09 Fluorine". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "53 Iodine". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "03 Lithium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "48 Cadmium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "34 Selenium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "14 Silicon". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "13 Aluminium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "35 Bromine". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "90 Thorium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "57 Lanthanum". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "68 Erbium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "65 Terbium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "44 Ruthenium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "55 Caesium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ Caesium
- ^ "37 Rubidium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "81 Thallium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "49 Indium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "02 Helium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "31 Gallium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "70 Ytterbium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "67 Holmium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "69 Thulium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "21 Scandium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "62 Samarium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "64 Gadolinium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ أ ب "59 Praseodymium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ أ ب "60 Neodymium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "32 Germanium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "18 Argon". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ أ ب "10 Neon". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "54 Xenon". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "84 Polonium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "88 Radium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ Partington, J. R. (May 1957). "Discovery of Radon". Nature. 179 (4566): 912. Bibcode:1957Natur.179..912P. doi:10.1038/179912a0.
- ^ Ramsay, W.; Gray, R. W. (1910). "La densité de l'emanation du radium". Comptes rendus hebdomadaires des séances de l'Académie des sciences. 151: 126–128.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "89 Actinium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "63 Europium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "71 Lutetium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ http://www.maik.ru/abstract/radchem/0/radchem0535_abstract.pdf
- ^ "72 Hafnium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ Noddack, W.; Tacke, I.; Berg, O (1925). "Die Ekamangane". Naturwissenschaften. 13 (26): 567. Bibcode:1925NW.....13..567.. doi:10.1007/BF01558746.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "91 Protactinium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ Emsley, John (2001). Nature's Building Blocks ((Hardcover, First Edition) ed.). Oxford University Press. p. 347. ISBN 0-19-850340-7.
- ^ "43 Technetium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ History of the Origin of the Chemical Elements and Their Discoverers, Individual Element Names and History, "Technetium"
- ^ "87 Francium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ Adloff, Jean-Pierre; Kaufman, George B. (2005-09-25). Francium (Atomic Number 87), the Last Discovered Natural Element. The Chemical Educator 10 (5). [2007-03-26]
- ^ "85 Astatine". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ Close, Frank E. (2004). Particle Physics: A Very Short Introduction. Oxford University Press. p. 2. ISBN 978-0-19-280434-1.
- ^ "93 Neptunium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "94 Plutonium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "95 Americium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "96 Curium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "97 Berkelium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "98 Californium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "99 Einsteinium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "100 Fermium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "101 Mendelevium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "102 Nobelium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "103 Lawrencium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "104 Rutherfordium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "105 Dubnium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "106 Seaborgium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "107 Bohrium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "109 Meitnerium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "108 Hassium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "110 Darmstadtium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "111 Roentgenium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ "112 Copernicium". Elements.vanderkrogt.net. Retrieved 2009-07-17.
- ^ "Discovery of the Element with Atomic Number 112". www.iupac.org. 2009-06-26. Retrieved 2009-07-17.
- ^ Oganessian, Yu. Ts.; Utyonkov, V. K.; Lobanov, Yu. V.; Abdullin, F. Sh.; Polyakov, A. N.; Shirokovsky, I. V.; Tsyganov, Yu. S.; Gulbekian, G. G.; Bogomolov, S. L.; Gikal, B.; Mezentsev, A.; Iliev, S.; Subbotin, V.; Sukhov, A.; Buklanov, G.; Subotic, K.; Itkis, M.; Moody, K.; Wild, J.; Stoyer, N.; Stoyer, M.; Lougheed, R. (October 1999). "Synthesis of Superheavy Nuclei in the 48Ca + 244Pu Reaction". Physical Review Letters. 83 (16): 3154. Bibcode:1999PhRvL..83.3154O. doi:10.1103/PhysRevLett.83.3154.
- ^ Oganessian, Yu. Ts.; Utyonkov, V. K.; Lobanov, Yu. V.; Abdullin, F. Sh.; Polyakov, A. N.; Shirokovsky, I. V.; Tsyganov, Yu. S.; Gulbekian, G. G.; Bogomolov, S. L.; Gikal, B.; Mezentsev, A.; Iliev, S.; Subbotin, V.; Sukhov, A.; Ivanov, O.; Buklanov, G.; Subotic, K.; Itkis, M.; Moody, K.; Wild, J.; Stoyer, N.; Stoyer, M.; Lougheed, R.; Laue, C.; Karelin, Ye.; Tatarinov, A. (2000). "Observation of the decay of 292116". Physical Review C. 63: 011301. Bibcode:2001PhRvC..63a1301O. doi:10.1103/PhysRevC.63.011301.
- ^ Oganessian, Yu. Ts.; Utyonkov, V. K.; Lobanov, Yu. V.; Abdullin, F. Sh.; Polyakov, A. N.; Sagaidak, R. N.; Shirokovsky, I. V.; Tsyganov, Yu. S.; Voinov, A. A.; Gulbekian, G.; Bogomolov, S.; Gikal, B.; Mezentsev, A.; Iliev, S.; Subbotin, V.; Sukhov, A.; Subotic, K.; Zagrebaev, V.; Vostokin, G.; Itkis, M.; Moody, K.; Patin, J.; Shaughnessy, D.; Stoyer, M.; Stoyer, N.; Wilk, P.; Kenneally, J.; Landrum, J.; Wild, J.; Lougheed, R. (2006). "Synthesis of the isotopes of elements 118 and 116 in the 249Cf and 245Cm+48Ca fusion reactions". Physical Review C. 74 (4): 044602. Bibcode:2006PhRvC..74d4602O. doi:10.1103/PhysRevC.74.044602.
- ^ أ ب Oganessian, Yu. Ts.; Utyonkov, V. K.; Dmitriev, S. N.; Lobanov, Yu. V.; Itkis, M. G.; Polyakov, A. N.; Tsyganov, Yu. S.; Mezentsev, A. N.; Yeremin, A. V.; Voinov, A.; Sokol, E.; Gulbekian, G.; Bogomolov, S.; Iliev, S.; Subbotin, V.; Sukhov, A.; Buklanov, G.; Shishkin, S.; Chepygin, V.; Vostokin, G.; Aksenov, N.; Hussonnois, M.; Subotic, K.; Zagrebaev, V.; Moody, K.; Patin, J.; Wild, J.; Stoyer, M.; Stoyer, N.; et al. (2005). "Synthesis of elements 115 and 113 in the reaction 243Am + 48Ca". Physical Review C. 72 (3): 034611. Bibcode:2005PhRvC..72c4611O. doi:10.1103/PhysRevC.72.034611.
- ^ Oganessian, Yu. Ts.; Abdullin, F. Sh.; Bailey, P. D.; Benker, D. E.; Bennett, M. E.; Dmitriev, S. N.; Ezold, J. G.; Hamilton, J. H.; Henderson, R. A.; Itkis, M. G.; Lobanov, Yu. V.; Mezentsev, A. N.; Moody, K. J.; Nelson, S. L.; Polyakov, A. N.; Porter, C. E.; Ramayya, A. V.; Riley, F. D.; Roberto, J. B.; Ryabinin, M. A.; Rykaczewski, K. P.; Sagaidak, R. N.; Shaughnessy, D. A.; Shirokovsky, I. V.; Stoyer, M. A.; Subbotin, V. G.; Sudowe, R.; Sukhov, A. M.; Tsyganov, Yu. S.; et al. (April 2010). "Synthesis of a New Element with Atomic Number Z=117". Physical Review Letters. 104 (14): 142502. Bibcode:2010PhRvL.104n2502O. doi:10.1103/PhysRevLett.104.142502. PMID 20481935.
وصلات خارجية
- History of the Origin of the Chemical Elements and Their Discoverers Last updated by Boris Pritychenko on March 30, 2004
- History of Elements of the Periodic Table
- Timeline of Element Discoveries
- Discovery of the Elements - The Movie - YouTube (1:18)
- The History Of Metals Timeline. A timeline showing the discovery of metals and the development of metallurgy.
This article may include material from Wikimedia licensed under CC BY-SA 4.0. Please comply with the license terms.