پالاديوم
 | |||||||||||||||
| پلاديوم | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| المظهر | أبيض فضي | ||||||||||||||
| الوزن الذري العياري Ar°(Pd) | |||||||||||||||
| پلاديوم في الجدول الدوري | |||||||||||||||
| |||||||||||||||
| الرقم الذري (Z) | 46 | ||||||||||||||
| المجموعة | 10 | ||||||||||||||
| الدورة | period 5 | ||||||||||||||
| المستوى الفرعي | d-block | ||||||||||||||
| التوزيع الإلكتروني | [Kr] 4d10 | ||||||||||||||
| الإلكترونات بالغلاف | 2, 8, 18, 18 | ||||||||||||||
| الخصائص الطبيعية | |||||||||||||||
| الطور at د.ح.ض.ق | صلب | ||||||||||||||
| نقطة الانصهار | 1828.05 K (1554.9 °س، 2830.82 °F) | ||||||||||||||
| نقطة الغليان | 3236 K (2963 °س، 5365 °ف) | ||||||||||||||
| الكثافة (بالقرب من د.ح.غ.) | 12.023 ج/سم³ | ||||||||||||||
| حين يكون سائلاً (عند ن.إ.) | 10.38 ج/سم³ | ||||||||||||||
| حرارة الانصهار | 16.74 kJ/mol | ||||||||||||||
| حرارة التبخر | 358 kJ/mol | ||||||||||||||
| السعة الحرارية المولية | 25.98 J/(mol·K) | ||||||||||||||
ضغط البخار
| |||||||||||||||
| الخصائص الذرية | |||||||||||||||
| حالات الأكسدة | 0, +1, +2, +3, +4, +5, +6 | ||||||||||||||
| الكهرسلبية | مقياس پاولنگ: 2.20 | ||||||||||||||
| طاقات التأين |
| ||||||||||||||
| نصف القطر الذري | empirical: 137 pm | ||||||||||||||
| نصف قطر التكافؤ | 139±6 pm | ||||||||||||||
| نصف قطر ڤان در ڤالز | 163 pm | ||||||||||||||
| خصائص أخرى | |||||||||||||||
| البنية البلورية | مكعب متوسطن حول الوجه | ||||||||||||||
| سرعة الصوت قضيب رفيع | 3070 م/ث (عند 20 °س) | ||||||||||||||
| قضيب رفيع | 71.8 W/(m·K) | ||||||||||||||
| التمدد الحراري | 11.8 µm/(m⋅K) (عند 25 °س) | ||||||||||||||
| المقاومة الكهربائية | 105.4 nΩ⋅m (at 20 °C) | ||||||||||||||
| الترتيب المغناطيسي | مغناطيسية مسايرة[1] | ||||||||||||||
| معامل يونگ | 121 GPa | ||||||||||||||
| معامل القص | 44 GPa | ||||||||||||||
| معاير الحجم | 180 GPa | ||||||||||||||
| نسبة پواسون | 0.39 | ||||||||||||||
| صلادة موز | 4.75 | ||||||||||||||
| صلادة ڤيكرز | 400–600 MPa | ||||||||||||||
| صلادة برينل | 320–610 MPa | ||||||||||||||
| رقم كاس | 7440-05-3 | ||||||||||||||
| التاريخ | |||||||||||||||
| التسمية | على اسم الكويكب پالاس، الذي هو نفسه مسمى على اسم پالاس أثينا | ||||||||||||||
| الاكتشاف وأول عزل | وليام هايد ولاستون (1803) | ||||||||||||||
| نظائر الپلاديوم | |||||||||||||||
| قالب:جدول نظائر پلاديوم غير موجود | |||||||||||||||
الپلاديوم Palladium عنصر كيميائي من الجدول الدوري، ورمزه Pd ، ورقمه الذري 46. وهو فلز انتقالي نادر، من مجموعة البلاتين. ويشبه البلاتين كيميائياً. ويستخرج من خامي النحاس والنيكل. ويستخدم كعامل مساعد وفي صناعة المجوهرات. الپلاديوم كان قد اكتشفه وليام هايد ولاستون William Hyde Wollaston عام 1803.[3][4] He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). These have similar chemical properties, but palladium has the lowest melting point and is the least dense of them.
More than half the supply of palladium and its congener platinum is used in catalytic converters, which convert as much as 90% of the harmful gases in automobile exhaust (hydrocarbons, carbon monoxide, and nitrogen dioxide) into less noxious substances (nitrogen, carbon dioxide and water vapor). Palladium is also used in electronics, dentistry, medicine, hydrogen purification, chemical applications, groundwater treatment, and jewelry. Palladium is a key component of fuel cells, which react hydrogen with oxygen to produce electricity, heat, and water.
Ore deposits of palladium and other PGMs are rare. The most extensive deposits have been found in the norite belt of the Bushveld Igneous Complex covering the Transvaal Basin in South Africa; the Stillwater Complex in Montana, United States; the Sudbury Basin and Thunder Bay District of Ontario, Canada; and the Norilsk Complex in Russia. Recycling is also a source, mostly from scrapped catalytic converters. The numerous applications and limited supply sources result in considerable investment interest.
الطلب العالمي على الپلاديوم ارتفع من 100 طن في 1990 إلى نحو 300 طن في 2000. الانتاج العالمي من المناجم كان 222 طن متري في 2006 حسب بيانات USGS. معظم الپالاديوم يستخدم في catalytic converters صناعة السيارات.[5]
الخصائص
الپالاديوم هو معدن فضي أبيض طري يشبه الپلاتين. Palladium belongs to group 10 in the periodic table, but the configuration in the outermost electrons is in accordance with Hund's rule. Electrons that by the Madelung rule would be expected to occupy the 5s instead fill the 4d orbitals, as it is more energetically favorable to have a completely filled 4d10 shell instead of the 5s2 4d8 configuration.
| Z | العنصر | No. of electrons/shell |
|---|---|---|
| 28 | نيكل | 2, 8, 16, 2 (or 2, 8, 17, 1) |
| 46 | پلاديوم | 2, 8, 18, 18 |
| 78 | پلاتين | 2, 8, 18, 32, 17, 1 |
| 110 | دارمشتاتيوم | 2, 8, 18, 32, 32, 16, 2 (متوقع) |
This 5s0 configuration, unique in period 5, makes palladium the heaviest element having only one incomplete electron shell, with all shells above it empty.
Palladium has the appearance of a soft silver-white metal that resembles platinum. It is the least dense and has the lowest melting point of the platinum group metals. It is soft and ductile when annealed and is greatly increased in strength and hardness when cold-worked. Palladium dissolves slowly in concentrated nitric acid, in hot, concentrated sulfuric acid, and when finely ground, in hydrochloric acid.[6] It dissolves readily at room temperature in aqua regia.
Palladium does not react with oxygen at standard temperature (and thus does not tarnish in air). Palladium heated to 800 °C will produce a layer of palladium(II) oxide (PdO). It may slowly develop a slight brownish coloration over time, likely due to the formation of a surface layer of its monoxide.
Palladium films with defects produced by alpha particle bombardment at low temperature exhibit superconductivity having Tc=3.2 K.[7]
النظائر
Naturally occurring palladium is composed of seven isotopes, six of which are stable. The most stable radioisotopes are 107Pd with a half-life of 6.5 million years (found in nature), 103Pd with 17 days, and 100Pd with 3.63 days. Eighteen other radioisotopes have been characterized with atomic weights ranging from 90.94948(64) u (91Pd) to 122.93426(64) u (123Pd).[8] These have half-lives of less than thirty minutes, except 101Pd (half-life: 8.47 hours), 109Pd (half-life: 13.7 hours), and 112Pd (half-life: 21 hours).[9]
For isotopes with atomic mass unit values less than that of the most abundant stable isotope, 106Pd, the primary decay mode is electron capture with the primary decay product being rhodium. The primary mode of decay for those isotopes of Pd with atomic mass greater than 106 is beta decay with the primary product of this decay being silver.[9]
Radiogenic 107Ag is a decay product of 107Pd and was first discovered in 1978[10] in the Santa Clara[11] meteorite of 1976. The discoverers suggest that the coalescence and differentiation of iron-cored small planets may have occurred 10 million years after a nucleosynthetic event. 107Pd versus Ag correlations observed in bodies, which have been melted since accretion of the Solar System, must reflect the presence of short-lived nuclides in the early Solar System.[12] 107 Pd is also produced as a fission product in spontaneous or induced fission of 235 U. As it is not very mobile in the environment and has a relatively low decay energy, 107 Pd is usually considered to be among the less concerning of the long-lived fission products.
المركبات
مركبات الپلاديوم تتواجد أساساً في حالات الأكسدة 0 و +2 oxidation state. Other less common states are also recognized. Generally the compounds of palladium are more similar to those of platinum than those of any other element.
 |
 |
پلاديوم(II)
كلوريد الپلاديوم(II) هو المادة البادئة الرئيسية لمركبات الپلاديوم الأخرى. It arises by the reaction of palladium with chlorine. It is used to prepare heterogeneous palladium catalysts such as palladium on barium sulfate, palladium on carbon, and palladium chloride on carbon.[13] Solutions of PdCl2 in nitric acid react with acetic acid to give palladium(II) acetate, also a versatile reagent. PdCl2 reacts with ligands (L) to give square planar complexes of the type PdCl2L2. One example of such complexes is the benzonitrile derivative PdX2(PhCN)2.[14][15]
- PdCl2 + 2 L → PdCl2L2 (L = PhCN, PPh3, NH3, etc)
The complex bis(triphenylphosphine)palladium(II) dichloride is a useful catalyst.[16]
پلاديوم(0)
Palladium forms a range of zerovalent complexes with the formula PdL4, PdL3 and PdL2. For example, reduction of a mixture of PdCl2(PPh3)2 and PPh3 gives tetrakis(triphenylphosphine)palladium(0):[17]
- 2 PdCl2(PPh3)2 + 4 PPh3 + 5 N2H4 → 2 Pd(PPh3)4 + N2 + 4 N2H5+Cl−
Another major palladium(0) complex, tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3), is prepared by reducing sodium tetrachloropalladate in the presence of dibenzylideneacetone.[18]
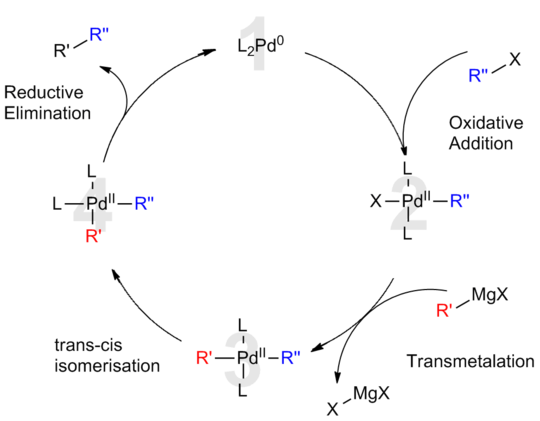
Palladium(0), as well as palladium(II), are catalysts in coupling reactions, as has been recognized by the 2010 Nobel Prize in Chemistry to Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki. Such reactions are widely practiced for the synthesis of fine chemicals. Prominent coupling reactions include the Heck, Suzuki, Sonogashira coupling, Stille reactions, and the Kumada coupling. Palladium(II) acetate, tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4, and tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) serve either as catalysts or precatalysts.[19]
حالات أكسدة أخرى
بالرغم من أن مركبات Pd(IV) هم نسبياً نادرون، فأحد الأمثلة هو sodium hexachloropalladate(IV), Na2[PdCl6]. A few compounds of palladium(III) are also known.[20] Palladium(VI) was claimed in 2002,[21][22] but subsequently disproven.[23][24]
Mixed valence palladium complexes exist, e.g. Pd4(CO)4(OAc)4Pd(acac)2 forms an infinite Pd chain structure, with alternatively interconnected Pd4(CO)4(OAc)4 and Pd(acac)2 units.[25]
تاريخ
الپلاديوم كان قد اكتشفه وليام هايد ولاستون William Hyde Wollaston في معمله في يوليو 1802، اختار اسمه في أغسطس من نفس العام.[26][27] وكان ولاستون قد سمّى هذا العنصر عام 1804 على اسم المذنّب پالاس Pallas, والذي كان قد اكتُشِف قبل عامين.[28] Wollaston purified a quantity of the material and offered it, without naming the discoverer, in a small shop in Soho in April 1803. After harsh criticism from Richard Chenevix that palladium is an alloy of platinum and mercury, Wollaston anonymously offered a reward of £20 for 20 grains of synthetic palladium alloy.[29] Chenevix received the Copley Medal in 1803 after he published his experiments on palladium. Wollaston published the discovery of rhodium in 1804 and mentions some of his work on palladium.[30][31] He disclosed that he was the discoverer of palladium in a publication in 1805.[29][32]
It was named by Wollaston in 1802 after the asteroid 2 Pallas, which had been discovered two months earlier.[6] Wollaston found palladium in crude platinum ore from South America by dissolving the ore in aqua regia, neutralizing the solution with sodium hydroxide, and precipitating platinum as ammonium chloroplatinate with ammonium chloride. He added mercuric cyanide to form the compound palladium(II) cyanide, which was heated to extract palladium metal.[30]
Palladium chloride was at one time prescribed as a tuberculosis treatment at the rate of 0.065 g per day (approximately one milligram per kilogram of body weight). This treatment had many negative side-effects, and was later replaced by more effective drugs.[33]
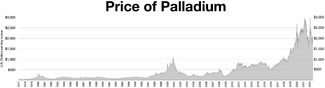
Most palladium is used for catalytic converters in the automobile industry.[34] In the run up to year 2000, the Russian supply of palladium to the global market was repeatedly delayed and disrupted; for political reasons, the export quota was not granted on time.[35] The ensuing market panic drove the price to an all-time high of $1،340 لكل أونصة ترويسية ($43/g) in January 2001.[36] Around that time, the Ford Motor Company, fearing that automobile production would be disrupted by a palladium shortage, stockpiled the metal. When prices fell in early 2001, Ford lost nearly US$1 billion.[37]
World demand for palladium increased from 100 tons in 1990 to nearly 300 tons in 2000. The global production of palladium from mines was 222 tonnes in 2006 according to the United States Geological Survey.[38] Many were concerned about a steady supply of palladium in the wake of Russia's annexation of Crimea, partly as sanctions could hamper Russian palladium exports; any restrictions on Russian palladium exports could have exacerbated what was already expected to be a large palladium deficit in 2014.[39] Those concerns pushed palladium prices to their highest level since 2001.[40] In September 2014 they soared above the $900 per ounce mark. In 2016 however palladium cost around $614 per ounce as Russia managed to maintain stable supplies.[41] In January 2019 palladium futures climbed past $1,344 per ounce for the first time on record, mainly due to the strong demand from the automotive industry.[42] Palladium reached $2،024.64 لكل أونصة ترويسية ($65.094/g) on 6 January 2020, passing $2,000 per troy ounce the first time.[43] The price rose above $3,000 per troy ounce in May 2021 and March 2022.[44]
التواجد
ببلوغ الإنتاج الإجمالي للبالاديوم 210,000 كيلوجرام في 2022، كانت روسيا المنتج الأكبر بحصة 88,000 كيلوجرام، تتبعها جنوب أفريقيا وكندا والولايات المتحدة، وزيمبابوى.[45] Russia's company Norilsk Nickel ranks first among the largest palladium producers globally, accounting for 39% of the world's production.[46]
Palladium can be found as a free metal alloyed with gold and other platinum-group metals in placer deposits of the Ural Mountains, Australia, Ethiopia, North and South America. For the production of palladium, these deposits play only a minor role. The most important commercial sources are nickel-copper deposits found in the Sudbury Basin, Ontario, and the Norilsk–Talnakh deposits in Siberia. The other large deposit is the Merensky Reef platinum group metals deposit within the Bushveld Igneous Complex South Africa. The Stillwater igneous complex of Montana and the Roby zone ore body of the Lac des Îles igneous complex of Ontario are the two other sources of palladium in Canada and the United States.[38][47] Palladium is found in the rare minerals cooperite[48] and polarite.[49] Many more Pd minerals are known, but all of them are very rare.[50]
Palladium is also produced in nuclear fission reactors and can be extracted from spent nuclear fuel (see synthesis of precious metals), though this source for palladium is not used. None of the existing nuclear reprocessing facilities are equipped to extract palladium from the high-level radioactive waste.[51] A complication for the recovery of Palladium in spent fuel is the presence of 107 Pd, a slightly radioactive long-lived fission product. Depending on end use, the radioactivity contributed by the 107 Pd might make the recovered Palladium unusable without a costly step of isotope separation.
الاستخدامات


The largest use of palladium today is in catalytic converters.[52] Palladium is also used in jewelry, dentistry,[52][53] watch making, blood sugar test strips, aircraft spark plugs, surgical instruments, and electrical contacts.[54] Palladium is also used to make professional transverse (concert or classical) flutes.[55] As a commodity, palladium bullion has ISO currency codes of XPD and 964. Palladium is one of only four metals to have such codes, the others being gold, silver and platinum.[56] Because it absorbs hydrogen, palladium is a key component of the controversial cold fusion experiments that began in 1989.
الحفز
When it is finely divided, as with palladium on carbon, palladium forms a versatile catalyst; it speeds heterogeneous catalytic processes like hydrogenation, dehydrogenation, and petroleum cracking. Palladium is also essential to the Lindlar catalyst, also called Lindlar's Palladium.[57] A large number of carbon–carbon bonding reactions in organic chemistry are facilitated by palladium compound catalysts. For example:
- Heck reaction
- Suzuki coupling
- Tsuji-Trost reactions
- Wacker process
- Negishi reaction
- Stille coupling
- Sonogashira coupling
(See palladium compounds and palladium-catalyzed coupling reactions.)
When dispersed on conductive materials, palladium is an excellent electrocatalyst for oxidation of primary alcohols in alkaline media.[58] Palladium is also a versatile metal for homogeneous catalysis, used in combination with a broad variety of ligands for highly selective chemical transformations.
In 2010, palladium-catalysed organic reactions were recognized by the Nobel Prize in Chemistry. A 2008 study showed that palladium is an effective catalyst for carbon-fluoride bonds.[59]
Palladium catalysis is primarily employed in organic chemistry and industrial applications, although its use is growing as a tool for synthetic biology; in 2017, effective in vivo catalytic activity of palladium nanoparticles was demonstrated in mammals to treat disease.[60]
الإلكترونيات
The second greatest application of palladium in electronics is in multilayer ceramic capacitors[61] in which palladium (and palladium-silver alloy) is used for electrodes.[52] Palladium (sometimes alloyed with nickel) is used for component and connector plating in consumer electronics[62][63] and in soldering materials. The electronic sector consumed 1.07 million troy ounces (33.2 tonnes) of palladium in 2006, according to a Johnson Matthey report.[64]
التكنولوجيا
Hydrogen easily diffuses through heated palladium,[6] and membrane reactors with Pd membranes are used in the production of high purity hydrogen.[65] Palladium is used in palladium-hydrogen electrodes in electrochemical studies. Palladium(II) chloride readily catalyzes carbon monoxide gas to carbon dioxide and is useful in carbon monoxide detectors.[66]
تخزين الهيدروجين
Palladium readily absorbs hydrogen at room temperatures, forming palladium hydride PdHx with x less than 1.[67] While this property is common to many transition metals, palladium has a uniquely high absorption capacity and does not lose its ductility until x approaches 1.[68] This property has been investigated in designing an efficient, inexpensive, and safe hydrogen fuel storage medium, though palladium itself is currently prohibitively expensive for this purpose.[69] The content of hydrogen in palladium can be linked to magnetic susceptibility, which decreases with the increase of hydrogen and becomes zero for PdH0.62. At any higher ratio, the solid solution becomes diamagnetic.[70]
طب الأسنان
Palladium is used in small amounts (about 0.5%) in some alloys of dental amalgam to decrease corrosion and increase the metallic lustre of the final restoration.[71]
المجوهرات
Palladium has been used as a precious metal in jewelry since 1939 as an alternative to platinum in the alloys called "white gold", where the naturally white color of palladium does not require rhodium plating. Palladium is much less dense than platinum. Similar to gold, palladium can be beaten into leaf as thin as 100 nm (1⁄250,000 in).[6] Unlike platinum, palladium may discolor at temperatures above 400 °C (752 °F);[72] it is relatively brittle.[مطلوب توضيح]
Prior to 2004, the principal use of palladium in jewelry was the manufacture of white gold. Palladium is one of the three most popular alloying metals in white gold (nickel and silver can also be used).[52] Palladium-gold is more expensive than nickel-gold, but seldom causes allergic reactions (though certain cross-allergies with nickel may occur).[73]
When platinum became a strategic resource during World War II, many jewelry bands were made out of palladium. Palladium was little used in jewelry because of the technical difficulty of casting. With the casting problem resolved[citation needed] the use of palladium in jewelry increased, originally because platinum increased in price while the price of palladium decreased.[74] In early 2004, when gold and platinum prices rose steeply, China began fabricating volumes of palladium jewelry, consuming 37 tonnes in 2005. Subsequent changes in the relative price of platinum lowered demand for palladium to 17.4 tonnes in 2009.[75][76] Demand for palladium as a catalyst has increased the price of palladium to about 50% higher than that of platinum in January 2019.[77]
In January 2010, hallmarks for palladium were introduced by assay offices in the United Kingdom, and hallmarking became mandatory for all jewelry advertising pure or alloyed palladium. Articles can be marked as 500, 950, or 999 parts of palladium per thousand of the alloy.
Fountain pen nibs made from gold are sometimes plated with palladium when a silver (rather than gold) appearance is desired. Sheaffer has used palladium plating for decades, either as an accent on otherwise gold nibs or covering the gold completely.
التصوير
In the platinotype printing process, photographers make fine-art black-and-white prints using platinum or palladium salts. Often used with platinum, palladium provides an alternative to silver.[78]
الأبحاث
الانصهار البارد
Palladium plays an important role in the ongoing research into cold-fusion energy.
الزجاج الفلزي فائق المتانة
Research is being done to develop metallic glass as a microalloy featuring palladium, a metal with a high "bulk-to-shear" stiffness ratio that counteracts the intrinsic brittleness of glassy materials. The initial samples of the new metallic glass were microalloys of palladium with phosphorous, silicon and germanium that yielded glass rods approximately one millimeter in diameter. Adding silver to the mix enabled the Cal Tech researchers to expand the thickness of the glass rods to six millimeters.[79]
البدائل
Pseudo palladium (RhAg) is a binary alloy consisting of equal parts of rhodium (atomic number 45) and silver (atomic number 47). This alloy exhibits properties of palladium (atomic number 46).[80]
الأثر على الصحة
السمية
| المخاطر | |
|---|---|
| ن.م.ع. مخطط تصويري | 
|
| ن.م.ع. كلمة الاشارة | Warning |
| H317 | |
| P261, P273, P280, P302+P352, P321, P333+P313, P363, P501[81] | |
| NFPA 704 (معيـَّن النار) | |
Palladium is a metal with low toxicity as conventionally measured (e.g. LD50). Recent research on the mechanism of palladium toxicity suggests high toxicity if measured on a longer timeframe and at the cellular level in the liver and kidney.[82] Mitochondria appear to have a key role in palladium toxicity via mitochondrial membrane potential collapse and depletion of the cellular glutathione (GSH) level. Until that recent work, it had been thought that palladium was poorly absorbed by the human body when ingested. Plants such as the water hyacinth are killed by low levels of palladium salts, but most other plants tolerate it, although tests show that, at levels above 0.0003%, growth is affected. High doses of palladium could be poisonous; tests on rodents suggest it may be carcinogenic, though until the recent research cited above, no clear evidence indicated that the element harms humans.[83]
المحاذير
Like other platinum-group metals, bulk Pd is quite inert. Although contact dermatitis has been reported, data on the effects are limited. It has been shown that people with an allergic reaction to palladium also react to nickel, making it advisable to avoid the use of dental alloys containing palladium on those so allergic.[34][84][85][86][87]
Some palladium is emitted with the exhaust gases of cars with catalytic converters. Between 4 and 108 ng/km of palladium particulate is released by such cars, while the total uptake from food is estimated to be less than 2 µg per person a day. The second possible source of palladium is dental restoration, from which the uptake of palladium is estimated to be less than 15 µg per person per day. People working with palladium or its compounds might have a considerably greater uptake. For soluble compounds such as palladium chloride, 99% is eliminated from the body within 3 days.[34]
The median lethal dose (LD50) of soluble palladium compounds in mice is 200 mg/kg for oral and 5 mg/kg for intravenous administration.[34]
الپلاديوم كاستثمار
Global palladium sales were 8.84 million ounces in 2017[88] of which 86% was used in the manufacturing of automotive catalytic converters, followed by industrial, jewelry, and investment usages.[89] Palladium is a chemical element first discovered in 1803, and since the 1980s, its major commercial application has been in the automotive industry.[90] More than 75% of global platinum and 40% of palladium are mined in South Africa. Russia's mining company, Norilsk Nickel, produces another 44% of palladium, with US and Canada-based mines producing most of the rest.
The price for palladium reached an all-time high of $2,981.40 per ounce on May 3, 2021[91][92] driven mainly on speculation of the catalytic converter demand from the automobile industry. Palladium is traded in the spot market with the code "XPD". When settled in USD, the code is "XPDUSD". A later surplus of the metal was caused by the Russian government selling stockpiles from the Soviet Era, at a rate of about 1.6 to 2 million ounces a year. The amount and status of this stockpile are a state secret.
During the Russo-Ukrainian War in March 2022, prices for palladium increased 13 percent since the first of March. Russia is the primary supplier to Europe and the country supplies 37 percent of the global production.[93]
منتجو الپلاديوم
- Norilsk Nickel (MCX: GMKN, LSE: MNOD), palladium powder and ingots.
- North American Palladium (NYSE: PAL), Canada's largest producer of palladium operating the Lac des Iles palladium mine near Thunder Bay, Ontario.
- Stillwater Mining (NYSE: SWC), a major North American palladium miner in Montana.[90]
المنتجات المتداولة عبر البورصة
WisdomTree Physical Palladium (LSE: PHPD) is backed by allocated palladium bullion and was the world's first palladium ETF. It is listed on the London Stock Exchange as PHPD,[94] Xetra Trading System, Euronext and Milan. ETFS Physical Palladium Shares (NYSE: PALL) is an ETF traded on the New York Stock Exchange.
القوالب والعملات والقضبان
A traditional way of investing in palladium is buying bullion coins and bars made of palladium. Available palladium coins include the Canadian Palladium Maple Leaf, the Chinese Panda, and the American Palladium Eagle. The liquidity of direct palladium bullion investment is poorer than that of gold and silver because there is low circulation of palladium coins.[citation needed]
انظر أيضاً
المصادر
- ^ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Standard Atomic Weights 2013. Commission on Isotopic Abundances and Atomic Weights
- ^ Platinum Metals Review, Rhodium and Palladium - Events Surrounding Its Discovery, accessed 5 Feb 2007.
- ^ W. H. Wollaston (1804). "On a New Metal, Found in Crude Platina". Philosophical Transactions of the Royal Society of London. 94: 419-430.
- ^ J. Kielhorn, C. Melber, D. Keller, I. Mangelsdorf (2002). "Palladium – A review of exposure and effects to human health". International Journal of Hygiene and Environmental Health. 205 (6). doi:10.1078/1438-4639-00180.
{{cite journal}}: Text "pages 417-432" ignored (help)CS1 maint: multiple names: authors list (link) - ^ أ ب ت ث Hammond, C. R. (2004). "The Elements". Handbook of Chemistry and Physics (81st ed.). CRC press. ISBN 978-0-8493-0485-9.
- ^ B. Strizker, Phys. Rev. Lett., 42, 1769 (1979).
- ^ "Atomic Weights and Isotopic Compositions for Palladium (NIST)". Nist. 2009-08-23. Retrieved 12 November 2009.
- ^ أ ب Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A 729: 3–128, doi:, Bibcode: 2003NuPhA.729....3A, https://hal.archives-ouvertes.fr/in2p3-00020241/document
- ^ Kelly, W. R.; Gounelle, G. J.; Hutchison, R. (1978). "Evidence for the existence of 107Pd in the early solar system". Philosophical Transactions of the Royal Society of London, Series A. 359 (1787): 1079–1082. Bibcode:2001RSPTA.359.1991R. doi:10.1098/rsta.2001.0893. S2CID 120355895.
- ^ "Mexico's Meteorites" (PDF). mexicogemstones.com. Archived from the original (PDF) on 2006-05-06.
- ^ Chen, J. H.; Wasserburg, G. J. (1990). "The isotopic composition of Ag in meteorites and the presence of 107Pd in protoplanets". Geochimica et Cosmochimica Acta. 54 (6): 1729–1743. Bibcode:1990GeCoA..54.1729C. doi:10.1016/0016-7037(90)90404-9.
- ^ Mozingo, Ralph (1955). "Palladium Catalysts". Organic Syntheses; Collected Volumes, 3, pp. 685.
- ^ Anderson, Gordon K.; Lin, Minren; Sen, Ayusman; Gretz, Efi (1990). "Bis(Benzonitrile)Dichloro Complexes of Palladium and Platinum". Inorganic Syntheses. Inorganic Syntheses. 28: 60–63. doi:10.1002/9780470132593.ch13. ISBN 978-0-470-13259-3.
- ^ Zalevskaya, O. A; Vorob'eva, E. G; Dvornikova, I. A; Kuchin, A. V (2008). "Palladium complexes based on optically active terpene derivatives of ethylenediamine". Russian Journal of Coordination Chemistry. 34 (11): 855–857. doi:10.1134/S1070328408110110.
- ^ Miyaura, Norio; Suzuki, Akira (1993). "Palladium-catalyzed reaction of 1-alkenylboronates with vinylic halides: (1Z,3E)-1-Phenyl-1,3-octadiene". Organic Syntheses; Collected Volumes, 8, pp. 532.
- ^ Coulson, D. R.; Satek, L. C.; Grim, S. O. (1972). "23. Tetrakis(triphenylphosphine)palladium(0)". Inorg. Synth. Inorganic Syntheses. 13: 121–124. doi:10.1002/9780470132449.ch23. ISBN 978-0-470-13244-9.
- ^ Takahashi, Y; Ito, Ts; Sakai, S; Ishii, Y (1970). "A novel palladium(0) complex; bis(dibenzylideneacetone)palladium(0)". Journal of the Chemical Society D: Chemical Communications (17): 1065. doi:10.1039/C29700001065.
- ^ Crabtree, Robert H. (2009). "Application to Organic Synthesis". The Organometallic Chemistry of the Transition Metals. John Wiley and Sons. p. 392. ISBN 978-0-470-25762-3.
- ^ Powers, David C; Ritter, Tobias (2011). "Palladium(III) in Synthesis and Catalysis". Higher Oxidation State Organopalladium and Platinum Chemistry. Topics in Organometallic Chemistry. Vol. 35. pp. 129–156. doi:10.1007/978-3-642-17429-2_6. ISBN 978-3-642-17428-5. PMC 3066514. PMID 21461129.
- ^ Chen, W; Shimada, S; Tanaka, M (2002). "Synthesis and Structure of Formally Hexavalent Palladium Complexes". Science. 295 (5553): 308–310. doi:10.1126/science.1067027. PMID 11786638.
- ^ Crabtree, R. H (2002). "CHEMISTRY: A New Oxidation State for Pd?". Science. 295 (5553): 288–289. doi:10.1126/science.1067921. PMID 11786632.
- ^ Aullón, G; Lledós, A; Alvarez, S (2002). "Hexakis(silyl)palladium(VI) or palladium(II with eta2-disilane ligands?". Angewandte Chemie (International Ed. In English). 41 (11): 1956–9. PMID 19750645.
- ^ Sherer, E. C; Kinsinger, C. R; Kormos, B. L; Thompson, J. D; Cramer, C. J (2002). "Electronic structure and bonding in hexacoordinate silyl-palladium complexes". Angewandte Chemie (International Ed. In English). 41 (11): 1953–6. PMID 19750644.
- ^ Yin, Xi; Warren, Steven A; Pan, Yung-Tin; Tsao, Kai-Chieh; Gray, Danielle L; Bertke, Jeffery; Yang, Hong (2014). "A Motif for Infinite Metal Atom Wires". Angewandte Chemie International Edition. 53 (51): 14087–14091. doi:10.1002/anie.201408461. PMID 25319757.
- ^ Platinum Metals Review, Rhodium and Palladium - Events Surrounding Its Discovery, accessed 5 Feb 2007.
- ^ W. H. Wollaston (1804). "On a New Metal, Found in Crude Platina". Philosophical Transactions of the Royal Society of London. 94: 419-430.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةlanl - ^ أ ب Usselman, Melvyn (1978). "The Wollaston/Chenevix controversy over the elemental nature of palladium: A curious episode in the history of chemistry". Annals of Science. 35 (6): 551–579. doi:10.1080/00033797800200431.
- ^ أ ب Griffith, W. P. (2003). "Rhodium and Palladium – Events Surrounding Its Discovery". Platinum Metals Review. 47 (4): 175–183. Archived from the original on 4 July 2013. Retrieved 24 March 2005.
- ^ Wollaston, W. H. (1804). "On a New Metal, Found in Crude Platina". Philosophical Transactions of the Royal Society of London. 94: 419–430. doi:10.1098/rstl.1804.0019.
- ^ Wollaston, W. H. (1805). "On the Discovery of Palladium; With Observations on Other Substances Found with Platina". Philosophical Transactions of the Royal Society of London. 95: 316–330. doi:10.1098/rstl.1805.0024.
- ^ Garrett, Christine E.; Prasad, Kapa (2004). "The Art of Meeting Palladium Specifications in Active Pharmaceutical Ingredients Produced by Pd-Catalyzed Reactions". Advanced Synthesis & Catalysis. 346 (8): 889–900. doi:10.1002/adsc.200404071.
- ^ أ ب ت ث Kielhorn, Janet; Melber, Christine; Keller, Detlef; Mangelsdorf, Inge (2002). "Palladium – A review of exposure and effects to human health". International Journal of Hygiene and Environmental Health. 205 (6): 417–32. doi:10.1078/1438-4639-00180. PMID 12455264.
- ^ Williamson, Alan. "Russian PGM Stocks" (PDF). The LBMA Precious Metals Conference 2003. The London Bullion Market Association. Archived from the original (PDF) on 21 October 2013. Retrieved 2 October 2010.
- ^ "Historical Palladium Prices and Price Chart". InvestmentMine. Retrieved 2015-01-27.
- ^ "Ford fears first loss in a decade". BBC News. 16 January 2002. Retrieved 19 September 2008.
- ^ أ ب "Platinum-Group Metals" (PDF). Mineral Commodity Summaries. United States Geological Survey. January 2007.
- ^ Nat Rudarakanchana (2014-03-27). "Palladium Fund Launches in South Africa, As Russian Supply Fears Warm Prices". International Business Times.
- ^ Rosenfeld, Everett (2014-08-20). "The other commodity that's leaping on Ukraine war". CNBC. Retrieved 2018-01-29.
- ^ "Palladium Rally Is About More Than Just Autos". Bloomberg.com (in الإنجليزية). 2017-08-30. Retrieved 2018-01-29.
- ^ "Don't Expect Palladium Prices To Plunge | OilPrice.com". OilPrice.com (in الإنجليزية). Retrieved 2018-01-29.
- ^ "Gold soars as Middle East tensions brew perfect storm | Reuters". Reuters (in الإنجليزية). 6 January 2020. Retrieved 2020-01-06.
- ^ Patel, Brijesh (4 March 2022). "Palladium tops $3,000/oz as supply fears grow, gold jumps over 1%". Reuters.
- ^ Survey, U. S. Geological (2023). "Mineral commodity summaries 2023" (in الإنجليزية): 210.
{{cite journal}}: Cite journal requires|journal=(help) - ^ ""Norilsk Nickel" Group announces preliminary consolidated production results for 4 th quarter and full 2016, and production outl". Nornickel (in الإنجليزية الأمريكية). Archived from the original on 29 June 2018. Retrieved 2018-01-29.
- ^ "Platinum-Group Metals" (PDF). Mineral Yearbook 2007. United States Geological Survey. January 2007.
- ^ Verryn, Sabine M. C.; Merkle, Roland K. W. (1994). "Compositional variation of cooperite, braggite, and vysotskite from the Bushveld Complex". Mineralogical Magazine. 58 (2): 223–234. Bibcode:1994MinM...58..223V. CiteSeerX 10.1.1.610.640. doi:10.1180/minmag.1994.058.391.05. S2CID 53128786.
- ^ Genkin, A. D.; Evstigneeva, T. L. (1986). "Associations of platinum- group minerals of the Norilsk copper-nickel sulfide ores". Economic Geology. 81 (5): 1203–1212. doi:10.2113/gsecongeo.81.5.1203.
- ^ "Mindat.org - Mines, Minerals and More". www.mindat.org.
- ^ Kolarik, Zdenek; Renard, Edouard V. (2003). "Recovery of Value Fission Platinoids from Spent Nuclear Fuel. Part I PART I: General Considerations and Basic Chemistry" (PDF). Platinum Metals Review. 47 (2): 74–87.
- ^ أ ب ت ث "Palladium". United Nations Conference on Trade and Development. Archived from the original on 6 December 2006. Retrieved 5 February 2007.
- ^ Rushforth, Roy (2004). "Palladium in Restorative Dentistry: Superior Physical Properties make Palladium an Ideal Dental Metal". Platinum Metals Review. 48 (1).
- ^ Hesse, Rayner W. (2007). "palladium". Jewelry-making through history: an encyclopedia. Greenwood Publishing Group. p. 146. ISBN 978-0-313-33507-5.
- ^ Toff, Nancy (1996). The flute book: a complete guide for students and performers. Oxford University Press. p. 20. ISBN 978-0-19-510502-5.
- ^ Weithers, Timothy Martin (2006). "Precious Metals". Foreign exchange: a practical guide to the FX markets. p. 34. ISBN 978-0-471-73203-7.
- ^ Brown, William Henry; Foote, Christopher S; Iverson, Brent L (2009). "Catalytic reduction". Organic chemistry. Cengage Learning. p. 270. ISBN 978-0-495-38857-9.
- ^ Tsuji, Jiro (2004). Palladium reagents and catalysts: new perspectives for the 21st century. John Wiley and Sons. p. 90. ISBN 978-0-470-85032-9.
- ^ Drahl, Carmen (2008). "Palladium's Hidden Talent". Chemical & Engineering News. 86 (35): 53–56. doi:10.1021/cen-v086n035.p053.
- ^ Miller, Miles A; Askevold, Bjorn; Mikula, Hannes; Kohler, Rainer H; Pirovich, David; Weissleder, Ralph (2017). "Nano-palladium is a cellular catalyst for in vivo chemistry". Nature Communications. 8: 15906. doi:10.1038/ncomms15906. PMC 5510178. PMID 28699627.
- ^ Zogbi, Dennis (3 February 2003). "Shifting Supply and Demand for Palladium in MLCCs". TTI, Inc.
- ^ Mroczkowski, Robert S. (1998). Electronic connector handbook: theory and applications. McGraw-Hill Professional. pp. 3–. ISBN 978-0-07-041401-3.
- ^ Harper, Charles A. (1997). Passive electronic component handbook. McGraw-Hill Professional. pp. 580–. ISBN 978-0-07-026698-8.
- ^ Jollie, David (2007). "Platinum 2007" (PDF). Johnson Matthey. Archived from the original (PDF) on 2008-02-16.
- ^ Shu, J.; Grandjean, B. P. A.; Neste, A. Van; Kaliaguine, S. (1991). "Catalytic palladium-based membrane reactors: A review". The Canadian Journal of Chemical Engineering. 69 (5): 1036. doi:10.1002/cjce.5450690503.
- ^ Allen, T. H.; Root, W. S. (1955). "An improved palladium chloride method for the determination of carbon monoxide in blood". The Journal of Biological Chemistry. 216 (1): 319–323. PMID 13252031.
- ^ Manchester, F. D.; San-Martin, A.; Pitre, J. M. (1994). "The H-Pd (hydrogen-palladium) System". Journal of Phase Equilibria. 15: 62–83. doi:10.1007/BF02667685.
- ^ Greenwood, N. N. (1997). Chemistry of the Elements (2nd Edition ed.). Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
{{cite book}}:|edition=has extra text (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Grochala, Wojciech; Edwards, Peter P. (2004). "Thermal Decomposition of the Non-Interstitial Hydrides for the Storage and Production of Hydrogen". Chemical Reviews. 104 (3): 1283–316. doi:10.1021/cr030691s. PMID 15008624.
- ^ Mott, N. F. and Jones, H. (1958) The Theory of Properties of metals and alloys. Oxford University Press. ISBN 0-486-60456-X. p. 200
- ^ Colon, Pierre; Pradelle-Plasse, Nelly; Galland, Jacques (2003). "Evaluation of the long-term corrosion behavior of dental amalgams: influence of palladium addition and particle morphology". Dental Materials. 19 (3): 232–9. doi:10.1016/S0109-5641(02)00035-0. PMID 12628436.
- ^ Gupta, Dinesh C.; Langer, Paul H.; ASTM Committee F-1 on Electronics (1987). Emerging semiconductor technology: a symposium. ASTM International. pp. 273–. ISBN 978-0-8031-0459-4.
{{cite book}}: CS1 maint: numeric names: authors list (link) - ^ Hindsen, M.; Spiren, A.; Bruze, M. (2005). "Cross-reactivity between nickel and palladium demonstrated by systemic administration of nickel". Contact Dermatitis. 53 (1): 2–8. doi:10.1111/j.0105-1873.2005.00577.x. PMID 15982224.
- ^ Holmes, E. (13 February 2007). "Palladium, Platinum's Cheaper Sister, Makes a Bid for Love". Wall Street Journal (Eastern edition). pp. B.1.
- ^ "Platinum-Group Metals" (PDF). Mineral Yearbook 2009. United States Geological Survey. January 2007.
- ^ "Platinum-Group Metals" (PDF). Mineral Yearbook 2006. United States Geological Survey. January 2007.
- ^ "Johnson Matthey Base Prices". 2019. Retrieved 7 January 2019.
- ^ Ware, Mike (2005). "Book Review of : Photography in Platinum and Palladium". Platinum Metals Review. 49 (4): 190–195. doi:10.1595/147106705X70291.
- ^ "New Glass Stronger than Any Known Material". 2011-01-11.
- ^ Kusada, Kohei; Yamauchi, Miho; Kobayashi, Hirokazu; Kitagawa, Hiroshi; Kubota, Yoshiki (2010). "Hydrogen-Storage Properties of Solid-Solution Alloys of Immiscible Neighboring Elements with Pd". Journal of the American Chemical Society. 132 (45): 15896–8. doi:10.1021/ja107362z. PMID 20979361.
- ^ "Msds - 373192".
- ^ Hosseini et al, Metallomics, 2016,8, 252-259; DOI 10.1039/C5MT00249D
- ^ Emsley, John (2011). Nature's Building Blocks: An A-Z Guide to the Elements. Oxford University Press. pp. 384, 387. ISBN 978-0-19-960563-7.
- ^ Zereini, Fathi; Alt, Friedrich (2006). "Health Risk Potential of Palladium". Palladium emissions in the environment: analytical methods, environmental assessment and health effects. Springer Science & Business. pp. 549–563. ISBN 978-3-540-29219-7.
- ^ Wataha, J. C.; Hanks, C. T. (1996). "Biological effects of palladium and risk of using palladium in dental casting alloys". Journal of Oral Rehabilitation. 23 (5): 309–20. doi:10.1111/j.1365-2842.1996.tb00858.x. PMID 8736443.
- ^ Aberer, Werner; Holub, Henriette; Strohal, Robert; Slavicek, Rudolf (1993). "Palladium in dental alloys – the dermatologists' responsibility to warn?". Contact Dermatitis. 28 (3): 163–5. doi:10.1111/j.1600-0536.1993.tb03379.x. PMID 8462294.
- ^ Wataha, John C.; Shor, Kavita (2010). "Palladium alloys for biomedical devices". Expert Review of Medical Devices. 7 (4): 489–501. doi:10.1586/erd.10.25. PMID 20583886.
- ^ "Total palladium supply worldwide 2017 | Statistic". Statista (in الإنجليزية). Retrieved 2018-10-15.
- ^ "Global palladium demand distribution by application 2016 | Statistic". Statista (in الإنجليزية). Retrieved 2018-10-15.
- ^ أ ب "How to Invest in Palladium". elementinvesting.com. Retrieved 2015-04-28.
- ^ "Historical Palladium Charts and Data - London Fix".
- ^ "CPI Inflation Calculator". data.bls.gov. Retrieved 2018-08-13.
- ^ Staff, Writer (OilPrice.com) (2022-03-10). "Palladium Prices Are Soaring As Russian Sanctions Sting". Yahoo! Finance. OilPrice.com. Retrieved 2022-03-13.
- ^ "ETFS METAL PAL ETP price (PHPD)". London Stock Exchange.
وصلات خارجية
- CS1 errors: unrecognized parameter
- CS1 الإنجليزية الأمريكية-language sources (en-us)
- CS1 errors: unsupported parameter
- CS1 errors: extra text: edition
- CS1 maint: numeric names: authors list
- Short description is different from Wikidata
- Pages using infobox element with unknown parameters
- Articles with hatnote templates targeting a nonexistent page
- جميع الصفحات التي تحتاج تنظيف
- مقالات بالمعرفة تحتاج توضيح from July 2016
- Articles with unsourced statements from January 2019
- Chembox container only
- شركات مدرجة في بورصة لندن
- شركات مدرجة في بورصة نيويورك
- Articles with unsourced statements from August 2007
- پلاديوم
- عناصر كيميائية
- فلزات نبيلة
- فلزات انتقالية
- معادن نفيسة
- Native element minerals
- Chemical elements with face-centered cubic structure