لا فلز
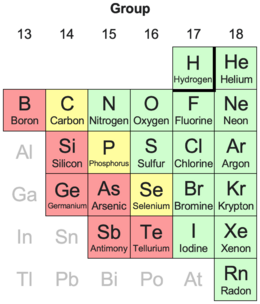
Extract of periodic table showing how often each element is classified as a nonmetal:
14 effectively always[n 1] 3 frequently[n 2] 6 sometimes (metalloids)[n 3]
Nearby metals are shown in a gray font.[n 4]
There is no precise definition of a nonmetal; which elements are counted as such varies.
Hydrogen is usually in group 1 (per the below full table) but can be in group 17 (per the above extract).[n 5]
| ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ |
| ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ ▉ |
اللا فلزات مثل الفلزات وأشباه الفلزات إحدى السلاسل الكيميائية ، وتتميز بخصائص معينة من ناحية التأين والترابط . وتنبع هذه الخواص من ان اللافلزات عالية السالبية الكهربية ، أى أنها تكتسب إلكترونات التكافؤ من الذرات الأخرى أسرع من فقدها .
اللافلزات مرتبة حسب الرقم الذري هى كالتالى :
- هيدروجين ( H )
- كربون ( C )
- نيتروجين ( N )
- أكسجين ( O )
- فلور ( F )
- فسفور ( P )
- كبريت ( S )
- كلور ( Cl )
- سيلينيوم ( Se )
- بروم ( Br )
- يود ( I )
- أستاتين ( At )
معظم اللافلزات توجد في أعلى الجانب الأيسر من الجدول الدوري ، فيما عدا الهيدروجين والذى يتم وضعه عادة في أعلى الجانب الأيمن مع الفلزات القلوية ، ولكنه يتصرف مثل اللافلزات في معظم الأحيان . اللا فلزات عكس الفلزات من حيث التوصيل الكهربى ، فهى إما عازلة أو شبه موصلة . ويمكك أن تقوم اللافلزات بتكوين رابطة أيونية مع الفلزات بإكتساب الإلكترونات ، أو تكون رابطة تساهمية مع لا فلزات أخرى . وتكون أكاسيد اللافلزات حمضية .
ورغم أنه يوجد 12 عنصر معروف من اللافلزات بالمقارنة بما يزيد عن 90 من الفلزات ، فإن اللافلزات يتكون منها معظم الأرض تقريبا ، وخاصة الطبقات الخارجية . وتتكون الكائنات الحية كلها تقريبا من اللافلزات . ويوجد كثير من اللافلزات ( الهيدروجين ، النيتروجين ، الأكسجين ، الفلور ، الكلور ، البروم ، اليود في حالة جزئي مزدوج الذرة ، و الباقى معظمه يوجد في حالة جزيئ عديد الذرات . قلَّما يصادف عنصر يتمتع بجميع خواص المعادن أو اللامعادن، فالعناصر كافة تقريباً تجمع بين خواص المعادن واللامعادن بنسب متفاوتة. وتقع المعادن النموذجية في القسم الأيسر والسفلي من الجدول الدوري، في حين تحتل اللامعادن النموذجية القسم الأيمن العلوي فيه. أما العناصر التي تمتد بين هذين القسمين فهي عناصر وسطية، إلا أنه ليس هناك حدود واضحة تفصل بين هذه المناطق الثلاث. فالهدروجين، على سبيل المثال، يصنَّف لامعدناً، إذا اعتبرت حالته الغازية ووزنه النوعي وتحوّله إلى شوارد سالبة في الهدريدات. إلا أنه يمكن تصنيفه معدناً بالنظر إلى ناقليته الجيدة للحرارة وتشكيله أيونات موجبة في محاليل الحموض. والأنتموان Sb صلب في الدرجة العادية من الحرارة، ويتمتع ببريق معدني إلا أنه هش لا يقبل الطرق والسحب، ويكوّن مع الهدروجين، مثل اللامعادن، مركباً طياراً ذا صيغة محددة SbH3.
والعناصر الوسطية، القريبة من الخط المنكسر بخاصة، تتمتع بخواص المعادن واللامعادن في الوقت نفسه، ويطلق على هذه العناصر اسم أشباه المعادن metalloids وهي: البور B، السيلكون Si، الجرمانيوم Ge، الزرنيخ As، التلوريوم Te. وأشباه المعادن تشبه، نوعاً ما، اللامعادن في خواصها الفيزيائية والكيمياوية.
لعل من الأفضل بدلاً من تقسيم العناصر إلى معادن ولامعادن شرح الخواص المعدنية[ر:المعدن] والخواص اللامعدنية. فإذا أمكن إهمال الخواص اللامعدنية مقابل الخواص المعدنية، عدّ العنصر معدناً نموذجياً، وبالعكس. فمن المعادن النموذجية المعادن القلوية مثل الصوديوم والبوتاسيوم على الرغم من وزنها النوعي المنخفض نسبياً، ومن اللامعادن الكربون على الرغم من ناقليته الجيدة للكهرباء وارتفاع درجة انصهاره.
الخواص العامة
الطبيعية
of some nonmetallic elements
إن ما يميز الحالة المعدنية من اللامعدنية هو طبيعة الرابطة وشكل البنية البلورية، أما جميع الخواص الأخرى الملازمة للحالة المعدنية كالبريق المعدني والخواص الحرارية والكهربائية واللدونة فهي نتائج لطبيعة الرابطة المعدنية والبنية البلورية المعدنية.
اللامعادن، من حيث المظهر، غازات أو أجسام صلبة درجات انصهارها منخفضة، مثال ذلك الهالوجينات، وهي عناصر الفصيلة VII A التي تعدّ لامعادن نموذجية. فالفلور F2 والكلور Cl2 غازان، البروم Br2 سائل، واليودI2 صلب درجة انصهاره 113.75 ْس.
تتفق الرابطة المشتركة المميزة للاّمعادن مع الرابطة المعدنية من حيث الطبيعة إذ إن كلاً من الرابطتين يتولّد من اشتراك الذرات فيما بينها بإلكترونات التكافؤ، إلا أن الرابطة المشتركة رابطة متموضعة localized، تنشأ بين ذرتين متجاورتين وتبقى الإلكترونات المشتركة في جوارهما المباشر، مرتبطة بهما ارتباطاً متيناً، وهذه الرابطة المتينة بين ذرات الجسم الصلب لا تسمح بانزلاق الذرات بعضها حول بعض من دون تفكك الرابطة وتخرب بنيان هذا الجسم. وهذا ما يفسر كون البلورات الملحية واللامعادن الصلبة ذات الروابط المشتركة هشة وقابلة للكسر، بخلاف المعادن التي تتمتع بلدونة كبيرة بفضل الرابطة المعدنية[ر:الرابطة الكيمياوية].
اللامعادن، بخلاف المعادن، ليست لدنة لأن الرابطة المشتركة فيها لا تتحرك بتحرك إلكترونات التكافؤ، لذلك لا يمكن لذراتها أن تتحرك بالنسبة لبعضها بعضاً من دون أن تؤدي الحركة إلى انفصام الرابطة المشتركة وتصدُّع البنيان اللامعدني، فهي لذلك لا تقبل السحب والطَرْق والمعالجات الميكانيكية الأخرى سواء بالطريقة الباردة أو بالطريقة الساخنة.
اللامعادن، بخلاف المعادن، رديئة النقل للحرارة والكهرباء، ويعود السبب في ذلك إلى طبيعة الرابطة فيها، إذ تعلّل الناقلية الحرارية في النظريات التقليدية بسرعة تحرك الإلكترونات واصطدامها بعضها ببعض اصطداماً مرناً، وهذا التحرك السريع غير ممكن في اللامعادن. أما الناقلية الكهربائية فتنشأ من سهولة تحرك الإلكترونات في حقل كهربائي مؤثر في المادة، حتى لو كان هذا الحقل ضعيفاً، فالإلكترونات تتحرك من دون انتظام في كتلة المعدن في حال غياب الحقل الكهربائي، أما عند تطبيق فرق كمون معين بين طرفي قطعة معدنية، فإنه ينشأ حقل كهربائي ينظم حركة الإلكترونات باتجاه القطب الموجب مما، يولِّد التيار الكهربائي.
وتعلَّل الناقلية الحرارية والكهربائية العالية في المعادن وانخفاضها في أشباه المعادن واللامعادن حسب النظريات الحديثة، بأن حالة الإلكترونات الخارجية في الذرة الحرة لعنصر تختلف عن حالتها عندما تجتمع ذرات العنصر لتكوّن كتلة متراصَّة، في حين تحافظ الإلكترونات الداخلية على حالتها الطاقية، ففي الذرات الحرة تكون الإلكترونات الخارجية (إلكترونات التكافؤ) ذات طاقات محدّدة، أو بتعبير آخر، تشغل سويات طاقة محددة. أما في كتلة العنصر، عندما تصبح هذه الإلكترونات خاضعة لتأثير عدد كبير من النوى، فإن السويات الطاقية الممكنة لهذه الإلكترونات تزداد حتى قيم أعلى، أو بتعبير آخر، تشغل هذه الإلكترونات منطقة طاقية عريضة محددة أعلى من سويات الطاقة الموافقة في الذرات الحرة، وبالتالي تصبح الطاقة اللازمة لإثارة الإلكترون الخارجي كي ينتقل إلى حالة طاقية أعلى أو لخروجه من الغلاف الإلكتروني أصغر بكثير، ويكفي لذلك حقل كهربائي صغير أو طاقة حرارية صغيرة. ويبقى مبدأ باولي[ر: الذرة] مطبقاً على توزيع الإلكترونات في المناطق الطاقية، كما في حالة الذرات الحرة. وإذا كانت المناطق الطاقية الممكنة مشغولة بصورة تامة فإن الانتقال من حالة إلى أخرى يصبح غير ممكن، وتكون الناقلية الكهربائية والحرارية معدومة (كما في حالة اللامعادن)، وكلما كان الفرق بين سويتي الطاقة الموافقتين للحالة العادية والحالة المثارة صغيراً (في حالة وجود مناطق طاقية شاغرة) كانت الناقلية الحرارية والكهربائية أجود.
وعلى هذا فإن ما يميز المعدن من اللامعدن هو أن المعدن يحوي سويات طاقية إلكترونية شاغرة وسويات طاقية تشغلها الإلكترونات المثارة بامتصاص طاقة صغيرة نسبياً، في حين لا توجد في كتلة اللامعدن النموذجي (أو في المواد العازلة) سويات طاقة شاغرة. وتكون السويات المثارة الممكنة عالية لايمكن بلوغها إلا بامتصاص طاقة كبيرة، فاللامعدن لا يصبح ناقلاً إلا في درجات الحرارة العالية.
أشباه المعادن تنقل التيار الكهربائي، ولكن ليس بجودة المعادن، فمعظمها أنصاف نواقل. وللسيلسيوم Si والجرمانيوم Ge، خاصة، تطبيقات واسعة لكونهما أنصاف نواقل. تكون أبخرة المعادن النموذجية غالباً في الحالة الذرية بخلاف اللامعادن التي تكون في الحالة الغازية متعددة الذرات (باستثناء الغازات الخاملة أحادية الذرة).
الكيميائية
| Aspect | Metals | Nonmetals |
|---|---|---|
| Electronegativity | Lower than nonmetals, with some exceptions[9] |
Moderate to very high |
| Chemical bonding | ||
| Seldom form covalent bonds |
Frequently form covalent bonds | |
| Metallic bonds (alloys) between metals |
Covalent bonds between nonmetals | |
| Ionic bonds between nonmetals and metals | ||
| Oxidation states |
Positive | Negative or positive |
| الأكاسيد | Basic in lower oxides; increasingly acidic in higher oxides |
Acidic; never basic[10] |
| In aqueous solution[11] |
Exist as cations | Exist as anions or oxyanions |
إن ما يميز اللامعادن من الوجهة الكيماوية، هو ميل ذراتها إلى ضم إلكترون أو عدة إلكترونات متحولة إلى شوارد (أيونات) سالبة، كما في الأملاح. ولهذا كانت قيم كمونات تشردها ionization potential كبيرة (وكمون التشرد هو الطاقة اللازمة لنزع أضعف الإلكترونات ارتباطاً بالذرة وهي بالحالة الغازية وتحولها إلى شاردة موجبة وهي بالحالة الغازية). فكمون تشرد الذرة، مقدراً بالكيلوجول/مول، للآزوت (لامعدن) 1402، وللصوديوم (معدن نشيط)496، وللكلسيوم (معدن أقل فعالية من الصوديوم) 599، وللزرنيخ 947.
يتحدد ميل الذرة لضم الإلكترونات، كمياً، بقيمة كمون المسرى النظامي normal electrode potential الموافقة لها (بالفولط)، وتنظَّم هذه القيم في جدول، هو الجدول الكهرحركي[ر. الكيمياء الكهربائية]. فكمون مسرى السيزيوم (معدن) Cs+/Cs يساوي -2.923 فولط وكمون مسرى الصوديوم الأقل فعالية من السيزيوم Na+/Na-2.71 فولط، وكمون مسرى الفلور 2F-/F2 يساوي +2.87 فولط وكمون مسرى الهدروجين 2H+/H2 يساوي الصفر. فالمعادن الفعالة تقع فوق الهدروجين بالجدول الكهرحركي، ولها قيم كمون مسرى سالبة. والعناصر التي تقع تحت الهدروجين بالجدول الكهرحركي أقل كهرجابية من الهدروجين، ولها قيم كمون مسرى موجبة.
لايقل جدول الكهرسلبية electronegativity أهمية عن الجدول الكهرحركي، إذ يمكن بالاستناد إليه تعيين طبيعة الرابطة بين ذرتين في مركَّب. والكهرسلبية هي ميل الذرة لجذب إلكترونات نحوها في الجزيء المعتدل، فالكلور، على سبيل المثال، أكثر كهرسلبية من الهدروجين، وهذا يعني أن الزوج الإلكتروني بين H وCl في المركَّب HCl، هذا الزوج يمضي وقتاً أطول حول الذرة الأكثر كهرسلبية، مما يكسب الكلور في الجزيء شحنة سلبية جزئية(δ-) ، ويكون الجزيء قطبياً أي إن له قطبين الهدروجين شحنته (δ-) والكلور شحنته (δ-) ثنائي القطب. وقد وضع الأميركي بولنغ Pauling مقياساً كمياً لميل الذرات لضم الإلكترونات لها، ورتب القيم في جدول . فأشد العناصر كهرسلبية الفلور كهرسلبيته تساوي 4.0 وأشد العناصر كهرجابية السيزيوم وقيمة كهرسلبيته 0.7، ووضعت فيما بعد جداول أخرى للكهرسلبية، والقيم في الجداول جميعها متقاربة والاختلاف بينها بسيط.
وتزداد الكهرسلبية في الدور بالجدول الدوري من اليسار إلى اليمين، وهي تنقص بالانتقال من أعلى الفصيلة إلى أسفلها.
وتتفاوت اللامعادن بنشاطها الكيمياوي فيما بينها تفاوتاً كبيراً، فالفلور، على سبيل المثال، شديد الفعالية، فهو يتحد، تقريباً، وعلى نحو فوري مع كافة العناصر، في حين أن الهليوم خامل جداً لا يتفاعل مع أي من العناصر أو مركباتها، ويستفيد منه الكيميائيون لخموله بتوفير وسط غير فعّال (خامل) داخل بعض الأجهزة.
ولا يجوز اقتصار الاعتماد على الجدول الكهرحركي وجدول الكهرسلبية في استنتاج الخواص الكيمياوية للعنصر، إذ يجب أن تؤخذ بعين الاعتبار عوامل أخرى مثل حالة العنصر، ونقاوة الكواشف الكيمياوية، وطبيعة نواتج التفاعل التي قد تسبب سلبية العنصر، أو تضعف من نشاطه الكيمياوي بانحلالها البطيء.
ومما يجدر ذكره أن الروابط في أكاسيد اللامعادن، مثل الآزوت (النتروجين) والفسفور والكبريت وأكاسيد العناصر ذات الكهرسلبية المتوسطة أو العالية، هي روابط مشتركة. وأكاسيد اللامعادن النموذجية ذات خواص حمضي.
التاريخ والخلفية والتبويب
الاكتشاف

Most nonmetals were discovered in the 18th and 19th centuries. Before then carbon, sulfur and antimony were known in antiquity; arsenic was discovered during the Middle Ages (by Albertus Magnus); and Hennig Brand isolated phosphorus from urine in 1669. Helium (1868) holds the distinction of being the only element not first discovered on Earth.[n 6] Radon is the most recently discovered nonmetal, being found only at the end of the 19th century.[13]
Chemistry- or physics-based techniques used in the isolation efforts were spectroscopy, fractional distillation, radiation detection, electrolysis, ore acidification, displacement reactions, combustion and heating; a few nonmetals occurred naturally as free elements
Of the noble gases, helium was detected via its yellow line in the coronal spectrum of the sun, and later by observing the bubbles escaping from uranite UO2 dissolved in acid. Neon through xenon were obtained via fractional distillation of air. Radon was first observed emanating from compounds of thorium, three years after Henri Becquerel's discovery of radiation in 1896.[14]
The nonmetal halogens were obtained from their halides via either electrolysis, adding an acid, or displacement. Some chemists died as a result of their experiments trying to isolate fluorine.[15]
Among the unclassified nonmetals, carbon was known (or produced) as charcoal, soot, graphite and diamond; nitrogen was observed in air from which oxygen had been removed; oxygen was obtained by heating mercurous oxide; phosphorus was liberated by heating ammonium sodium hydrogen phosphate (Na(NH4)HPO4), as found in urine;[16] sulfur occurred naturally as a free element; and selenium[n 7] was detected as a residue in sulfuric acid.[18]
Most of the elements commonly recognized as metalloids were isolated by heating their oxides (boron, silicon, arsenic, tellurium) or a sulfide (germanium).[13] Antimony was known in its native form as well as being attainable by heating its sulfide.[19]
أصل المفهوم
The distinction between metals and nonmetals arose, in a convoluted manner, from a crude recognition of different kinds of matter namely pure substances, mixtures, compounds and elements. Thus, matter could be divided into pure substances (such as salt, bicarb of soda, or sulfur) and mixtures (aqua regia, gunpowder, or bronze, for example) and pure substances eventually could be distinguished as compounds and elements.[20] "Metallic" elements then seemed to have broadly distinguishable attributes that other elements did not, such as their ability to conduct heat or for their "earths" (oxides) to form basic solutions in water, for example as occurred with quicklime (CaO).[21]
استخدام المصطلح
The term nonmetallic dates from as far back as 1566. In a medical treatise published that year, Loys de L’Aunay (a French doctor) mentioned the properties of plant substances from metallic and "non-metallic" lands.[22]
In early chemistry, Wilhelm Homberg (a German natural philosopher) referred to "non-metallic" sulfur in Des Essais de Chimie (1708).[23] He questioned the five-fold division of all matter into sulfur, mercury, salt, water and earth, as postulated by Étienne de Clave (1641) in New Philosophical Light of True Principles and Elements of Nature.[24] Homberg's approach represented "an important move toward the modern concept of an element".[25]
Lavoisier, in his "revolutionary"[26] 1789 work Traité élémentaire de chimie, published the first modern list of chemical elements in which he distinguished between gases, metals, nonmetals, and earths (heat resistant oxides).[27] In its first seventeen years, Lavoisier's work was republished in twenty-three editions in six languages, and "carried ... [his] new chemistry all over Europe and America."[28]
سمات مميـِّزة مقترحة
|
المتعلقة بالإلكترونات
|
In 1809, Humphry Davy's discovery of sodium and potassium "annihilated"[51] the line of demarcation between metals and nonmetals. Before then metals had been distinguished on the basis of their ponderousness or relatively high densities.[52] Sodium and potassium, on the other hand, floated on water and yet were clearly metals on the basis of their chemical behaviour.[53]
From as early as 1811, different properties—physical, chemical, and electron related—have been used in attempts to refine the distinction between metals and nonmetals. The accompanying table sets out 22 such properties, by type and date order.
Probably the most well-known property is that the electrical conductivity of a metal increases when temperature falls whereas that of a non-metal rises.[41] However this scheme does not work for plutonium, carbon, arsenic and antimony. Plutonium, which is a metal, increases its electrical conductivity when heated in the temperature range of around –175 to +125 °C.[54] Carbon, despite being widely regarded as a nonmetal, likewise increases its conductivity when heated.[55] Arsenic and antimony are sometimes classified as nonmetals yet act similarly to carbon.[56]
Emsley noted that, "No single property ... can be used to classify all the elements as either metals or nonmetals."[57] Kneen et al. suggested that the nonmetals could be discerned once a [single] criterion for metallicity had been chosen, adding that, "many arbitrary classifications are possible, most of which, if chosen reasonably, would be similar but not necessarily identical."[58] Jones, in contrast, observed that "classes are usually defined by more than two attributes".[59]
Johnson suggested that physical properties can best indicate the metallic or nonmetallic properties of an element, with the proviso that other properties will be needed in ambiguous cases. More specifically, he observed that all gaseous or nonconducting elements are nonmetals; solid nonmetals metals are hard and brittle or soft and crumbly whereas metals are usually malleable and ductile; and nonmetal oxides are acidic.[60]
Once a basis for distinguishing between the "two great classes of elements"[61] is established, the nonmetals are found to be those lacking the properties of metals,[62] to greater or lesser degrees.[63] Some authors further divide the elements into metals, metalloids, and nonmetals although Odberg argues that anything not a metal is, on categorisation grounds, a nonmetal.[64]
تطوير صفوف فرعية
A basic taxonomy of nonmetals was set out in 1844, by Alphonse Dupasquier, a French doctor, pharmacist and chemist.[65] To facilitate the study of nonmetals, he wrote:[66]
- They will be divided into four groups or sections, as in the following:
- Organogens O, N, H, C
- Sulphuroids S, Se, P
- Chloroides F, Cl, Br, I
- Boroids B, Si.
An echo of Dupasquier's fourfold classification is seen in the modern subclasses. The organogens and sulphuroids represent the set of unclassified nonmetals. Varying configurations of these seven nonmetals have been referred to as, for example, basic nonmetals;[67] biogens;[68] central nonmetals;[69] CHNOPS;[70] essential elements;[71] "nonmetals";[72][n 9] orphan nonmetals;[73] or redox nonmetals.[74] The chloroide nonmetals came to be independently referred to as halogens.[75] The boroid nonmetals expanded into the metalloids, starting from as early as 1864.[76] The noble gases, as a discrete grouping, were counted among the nonmetals from as early as 1900.[77]
مقارنة
Some properties of metals, and of metalloids, unclassified nonmetals, nonmetal halogens, and noble gases are summarized in the table.[n 10] Physical properties apply to elements in their most stable forms in ambient conditions, and are listed in loose order of ease of determination. Chemical properties are listed from general to descriptive, and then to specific. The dashed line around the metalloids denotes that, depending on the author, the elements involved may or may not be recognized as a distinct class or subclass of elements. Metals are included as a reference point.
Most properties show a left-to-right progression in metallic to nonmetallic character or average values. The periodic table can thus be indicatively divided into metals and nonmetals, with more or less distinct gradations seen among the nonmetals.[78]
| Physical property | Metals alkali, alkaline earth, lanthanide, actinide, transition, post-transition |
Metalloids boron, silicon, germanium, arsenic, antimony, tellurium |
Unclassified nonmetals hydrogen, carbon, nitrogen, phosphorus, oxygen, sulfur, selenium |
Nonmetal halogens fluorine, chlorine, bromine, iodine |
Noble gases helium, neon, argon, krypton, xenon, radon |
|---|---|---|---|---|---|
| Form and heft[79] |
|
|
|
|
|
| Appearance | lustrous[82] | lustrous[83] | colorless[88] | ||
| Elasticity | mostly malleable and ductile[82] (Hg is liquid) | brittle[83] | C, black P, S, Se brittle; all four have less stable non-brittle forms[89][n 11] | iodine is brittle[95] | not applicable |
| Electrical conductivity | good[n 12] |
|
|
|
poor[n 16] |
| Electronic structure[100] | metallic (Bi is a semimetal) | semimetal (As, Sb) or semiconductor |
|
semiconductor (I) or insulator | insulator |
| Chemical property | Metals alkali, alkaline earth, lanthanide, actinide, transition, post-transition |
Metalloids boron, silicon, germanium, arsenic, antimony, tellurium |
Unclassified nonmetals hydrogen, carbon, nitrogen, phosphorus, oxygen, sulfur, selenium |
Nonmetal halogens fluorine, chlorine, bromine, iodine |
Noble gases helium, neon, argon, krypton, xenon, radon |
| General chemical behavior |
|
weakly nonmetallic[n 17] | moderately nonmetallic[103] | strongly nonmetallic[104] | |
| Oxides | |||||
| Compounds with metals | alloys[82] or intermetallic compounds[122] | tend to form alloys or intermetallic compounds[123] | mainly ionic[125] | simple compounds in ambient conditions not known[n 20] | |
| Ionization energy (kJ mol−1)‡ (data page) |
|
|
|
|
|
| Electronegativity (Pauling)[n 21]‡ (data page) |
|
|
|
|
|
| † Hydrogen can also form alloy-like hydrides[128] ‡ The labels low, moderate, high, and very high are arbitrarily based on the value spans listed in the table | |||||
انظر أيضاً
- CHON (carbon, hydrogen, oxygen, nitrogen)
- List of nonmetal monographs
- Metallization pressure
- Period 1 elements (hydrogen, helium)
- Properties of nonmetals (and metalloids) by group
ملاحظات
- ^ H; N; O, S; F, Cl, Br, I; He, Ne, Ar, Kr, Xe, Rn[1]
- ^ C; P; Se.[1] On the other hand, these three elements were counted as metalloids in a survey of 194 lists of metalloids, 16, 10, and 46 times respectively.[2]
- ^ B; Si, Ge; As, Sb; Te[3][4]
- ^ Al, Ga, In, Tl; Sn, Pb; Bi; Po; At
- ^ Hydrogen has historically been placed over one or more of lithium, boron,[5] carbon, or fluorine;[6] or over no group at all; or over all main groups simultaneously, and therefore may or may not be adjacent to other nonmetals.[7]
- ^ How helium acquired the -ium suffix is explained in the following passage by its discoverer, William Lockyer: "I took upon myself the responsibility of coining the word helium ... I did not know whether the substance ... was a metal like calcium or a gas like hydrogen, but I did know that it behaved like hydrogen [being found in the sun] and that hydrogen, as Dumas had stated, behaved as a metal".[12]
- ^ Berzelius, who discovered selenium, thought it had the properties of a metal, combined with those of sulfur.[17]
- ^ The Goldhammer-Herzfeld ratio is roughly equal to the cube of the atomic radius divided by the molar volume.[32] More specifically, it is the ratio of the force holding an individual atom's outer electrons in place with the forces on the same electrons from interactions between the atoms in the solid or liquid element. When the interatomic forces are greater than, or equal to, the atomic force, outer electron itinerancy is indicated and metallic behaviour is predicted. Otherwise nonmetallic behaviour is anticipated.[33]
- ^ The quote marks are not found in the source; they are used here to make it clear that the source employs the word nonmetals as a formal term for the subset of chemical elements in question, rather than applying to nonmetals generally.
- ^ See also Properties of metals, metalloids and nonmetals, which treats metalloids as a class of their own
- ^ Carbon as exfoliated (expanded) graphite,[90] and as carbon nanotube wire;[91] phosphorus as white phosphorus (soft as wax, pliable and can be cut with a knife, at room temperature);[92] sulfur as plastic sulfur;[93] and selenium as selenium wires[94]
- ^ Metals have electrical conductivity values of from 6.9×103 S•cm−1 for manganese to 6.3×105 for silver.[96]
- ^ Metalloids have electrical conductivity values of from 1.5×10−6 S•cm−1 for boron to 3.9×104 for arsenic.[97]
- ^ Unclassified nonmetals have electrical conductivity values of from ca. 1×10−18 S•cm−1 for the elemental gases to 3±4 in graphite.[98]
- ^ The nonmetal halogens have electrical conductivity values of from ca. 1×10−18 S•cm−1 for F and Cl to 1.7×10−8 S•cm−1 for iodine.[98][99]
- ^ The elemental gases have electrical conductivity values of ca. 1×10−18 S•cm−1.[98]
- ^ They always give "compounds less acidic in character than the corresponding compounds of the [typical] nonmetals"[83]
- ^ Arsenic trioxide reacts with sulfur trioxide, forming arsenic "sulfate" As2(SO4)3.[111]
- ^ CO and N2O are "formally the anhydrides of formic and hyponitrous acid, respectively: CO + H2O → H2CO2 (HCOOH, formic acid); N2O + H2O → H2N2O2 (hyponitrous acid)".[116]
- ^ Disodium helide (Na2He) is a compound of helium and sodium that is stable at high pressures above 113 GPa. Argon forms an alloy with nickel, at 140 GPa and close to 1,500 K however at this pressure argon is no longer a noble gas.[126]
- ^ Values for the noble gases are from Rahm, Zeng and Hoffmann.[127]
المراجع
الهامش
- ^ أ ب Larrañaga, Lewis & Lewis 2016, p. 988
- ^ أ ب خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةVernon2013 - ^ Hérold 2006, pp. 149–50
- ^ أ ب Vernon 2020, p. 220
- ^ Luchinskii & Trifonov 1981, pp. 200–220
- ^ Jolly 1966, inside cover
- ^ Rayner-Canham 2020, p. 212
- ^ Kneen, Rogers & Simpson 1972, pp. 263‒264
- ^ Langley & Hattori 2014, p. 214
- ^ أ ب Abbott 1966, p. 18
- ^ Brown et al. 2014, p. 237
- ^ Labinger 2019, p. 305
- ^ أ ب خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةEmsley - ^ Emsley 2011, pp. 42–43, 219–220, 263–264, 341, 441–442, 596, 609
- ^ Emsley 2011, pp. 84, 128, 180–181, 247
- ^ Cook 1923, p. 124
- ^ Weeks 1945, p. 161
- ^ Emsley 2011, pp. 113, 363, 378, 477, 514–515
- ^ Weeks 1945, p. 22; Emsley 2011, p. 40
- ^ Klein 1994, p. 168
- ^ Lidin 1996, pp. 64‒65
- ^ de L'Aunay 1566, p. 7
- ^ Homberg 1708, p. 350; vide Kim 2000
- ^ de Clave 1641
- ^ Schlager & Lauer 2000, p. 370
- ^ Strathern 2000, p. 239
- ^ Criswell p. 1140
- ^ Salzberg 1991, p. 204
- ^ Kendall 1811, pp. 298–303
- ^ Brande 1821, p. 5
- ^ Edwards & Sienko 1983, pp. 691–96
- ^ Edwards & Sienko 1983, p. 693
- ^ Herzfeld 1927; Edwards 2000, pp. 100–03
- ^ Kubaschewski 1949, pp. 931–940
- ^ Remy 1956, p. 9
- ^ White 1962, p. 106: It makes a ringing sound when struck.
- ^ Johnson 1966, pp. 3–4
- ^ Horvath 1973, pp. 335–336
- ^ Rao & Ganguly 1986
- ^ Smith & Dwyer 1991, p. 65: The difference between melting point and boiling point.
- ^ أ ب Herman 1999, p. 702
- ^ Hill, Holman & Hulme 2017, p. 182: Atomic conductance is the electrical conductivity of one mole of a substance. It is equal to electrical conductivity divided by molar volume.
- ^ Suresh & Koga 2001, pp. 5940–5944
- ^ Johnson 2007, pp. 15–16
- ^ أ ب Edwards 2010, pp. 941–965
- ^ Povh & Rosin 2017, p. 131
- ^ Beach 1911
- ^ Stott 1956, pp. 100–102
- ^ Parish 1977, p. 178
- ^ Sanderson 1957, p. 229
- ^ Hare & Bache 1836, p. 310
- ^ Chambers 1743: "That which distinguishes metals from all other bodies ... is their heaviness ..."
- ^ Edwards 2000, p. 85
- ^ Russell & Lee 2005, p. 466
- ^ Atkins et al. 2006, pp. 320–21
- ^ Zhigal'skii & Jones 2003, p. 66
- ^ Emsley 1971, p. 1
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةKneen218 - ^ Jones 2010, p. 169
- ^ Johnson 1966, pp. 3–5, 15
- ^ Leach & Ewing 1966, p. 47
- ^ Brady & Senese 2009, p. 52
- ^ Zumdahl & DeCoste 2010, p. 92
- ^ Oderberg 2007, p. 97
- ^ Bertomeu-Sánchez, Garcia-Belmar & Bensaude-Vincent 2002, pp. 248–249
- ^ Dupasquier 1844, pp. 66–67
- ^ Williams 2007, pp. 1550–1561
- ^ Wächtershäuser 2014
- ^ Hengeveld & Fedonkin, pp. 181–226
- ^ Wakeman 1899, p. 562
- ^ Fraps 1913, p. 11
- ^ Parameswaran at al. 2020, p. 210
- ^ Knight 2002, p. 148
- ^ Fraústo da Silva & Williams 2001, p. 500
- ^ Berzelius 1832, pp. 248–276
- ^ The Chemical News 1864, p. 22
- ^ Renouf 1901, pp. 268
- ^ Vernon 2020, pp. 217–225
- ^ Tregarthen 2003, p. 10
- ^ Lewis 1993, pp. 28, 827
- ^ Lewis 1993, pp. 28, 813
- ^ أ ب ت خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةKneen - ^ أ ب ت ث Rochow 1966, p. 4
- ^ Wiberg 2001, p. 780; Emsley 2011, p. 397; Rochow 1966, pp. 23, 84
- ^ Kneen, Rogers & Simpson 1972, pp. 321, 404, 436
- ^ Kneen, Rogers & Simpson 1972, p. 439
- ^ Kneen, Rogers & Simpson 1972, p. 465
- ^ Kneen, Rogers & Simpson 1972, p. 308
- ^ Wiberg 2001, pp. 505, 681, 781; Glinka 1958, p. 355
- ^ Chung 1987, pp. 4190‒4198; Godfrin & Lauter 1995, pp. 216‒218
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةJanas - ^ Faraday 1853, p. 42; Holderness & Berry 1979, p. 255
- ^ Partington 1944, p. 405
- ^ Regnault 1853, p. 208
- ^ Wiberg 2001, p. 416
- ^ Desai, James & Ho 1984, p. 1160; Matula 1979, p. 1260
- ^ Schaefer 1968, p. 76; Carapella 1968, pp. 29‒32
- ^ أ ب ت Bogoroditskii & Pasynkov 1967, p. 77; Jenkins & Kawamura 1976, p. 88
- ^ Greenwood & Earnshaw 2002, p. 804
- ^ Keeler & Wothers 2013, p. 293
- ^ Kneen, Rogers & Simpson 1972, p. 264
- ^ Rayner-Canham 2018, p. 203
- ^ Welcher 2001, p. 3–32: "The elements change from ... metalloids, to moderately active nonmetals, to very active nonmetals, and to a noble gas."
- ^ Mackin 2014, p. 80
- ^ Johnson 1966, pp. 105–108
- ^ Stein 1969, pp. 5396‒5397; Pitzer 1975, pp. 760‒761
- ^ Porterfield 1993, p. 336
- ^ أ ب Rao 2002, p. 22
- ^ Wells 1984, p. 534
- ^ Atkins et al. 2006, pp. 8, 122–123
- ^ Wiberg 2001, p. 750
- ^ Sidorov 1960, pp. 599‒603
- ^ أ ب ت ث Puddephatt & Monaghan 1989, p. 59
- ^ أ ب Sanderson 1967, p. 172
- ^ أ ب Mingos 2019, p. 27
- ^ House 2008, p. 441
- ^ McMillan 2006, p. 823
- ^ King 1995, p. 182
- ^ Wiberg 2001, p. 399
- ^ Kläning & Appelman 1988, p. 3760
- ^ Ritter 2011, p. 10
- ^ Yamaguchi & Shirai 1996, p. 3
- ^ Vernon 2020, p. 223
- ^ Woodward et al. 1999, p. 134
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةCotton - ^ Dalton 2019
- ^ Rahm, Zeng & Hoffmann 2019, p. 345
- ^ Steudel 1977, p. 176
ببليوگرافيا
- Abbott D 1966, An Introduction to the Periodic Table, J. M. Dent & Sons, London
- Atkins PA 2001, The Periodic Kingdom: A Journey Into the Land of the Chemical Elements, Phoenix, London, ISBN 978-1-85799-449-0
- Atkins PA et al. 2006, Shriver & Atkins' Inorganic Chemistry, 4th ed., Oxford University Press, Oxford, ISBN 978-0-7167-4878-6
- Atkins PA & Overton T 2010, Shriver & Atkins' Inorganic Chemistry, 5th ed., Oxford University Press, Oxford, ISBN 978-0-19-923617-6
- Aylward G and Findlay T 2008, SI Chemical Data, 6th ed., John Wiley & Sons Australia, Milton, ISBN 978-0-470-81638-7
- Bailar JC et al. 1989, Chemistry, 3rd ed., Harcourt Brace Jovanovich, San Diego, ISBN 978-0-15-506456-0
- Barton AFM 2021, States of Matter, States of Mind, CRC Press, Boca Raton, ISBN 978-0-7503-0418-4
- Beach FC (ed.) 1911, The Americana: A universal reference library, vol. XIII, Mel–New, Metalloid, Scientific American Compiling Department, New York
- Benner SA, Ricardo A & Carrigan MA 2018, "Is there a common chemical model for life in the universe?", in Cleland CE & Bedau MA (eds.), The Nature of Life: Classical and Contemporary Perspectives from Philosophy and Science, Cambridge University Press, Cambridge, ISBN 978-1-108-72206-3
- Berger LI 1997, Semiconductor Materials, CRC Press, Boca Raton, ISBN 978-0-8493-8912-2
- Bertomeu-Sánchez JR, Garcia-Belmar A & Bensaude-Vincent B 2002, "Looking for an order of things: Textbooks and chemical classifications in nineteenth century France", Ambix, vol. 49, no. 3, DOI:10.1179/amb.2002.49.3.227
- Berzelius JJ & Bache AD 1832, "An essay on chemical nomenclature, prefixed to the treatise on chemistry", The American Journal of Science and Arts, vol. 22
- Bodner GM & Pardue HL 1993, Chemistry, An Experimental Science, John Wiley & Sons, New York, ISBN 0-471-59386-9
- Bogoroditskii NP & Pasynkov VV 1967, Radio and Electronic Materials, Iliffe Books, London
- Bohlmann R 1992, "Synthesis of halides", in Winterfeldt E (ed.), Heteroatom manipulation, Pergamon Press, Oxford, ISBN 978-0-08-091249-3
- Boise State University 2020, "Cost-effective manufacturing methods breathe new life into black phosphorus research", Micron School of Materials Science and Engineering, accessed July 9, 2021
- Borg RG & Dienes GJ 1992, The Physical Chemistry of Solids, Academic Press, Boston, ISBN 978-0-12-118420-9
- Boyd R 2011, "Selenium stories", Nature Chemistry, vol. 3, DOI:10.1038/nchem.1076
- Brady JE & Senese F 2009, Chemistry: The study of Matter and its Changes, 5th ed., John Wiley & Sons, New York, ISBN 978-0-470-57642-7
- Brande WT 1821, A Manual of Chemistry, vol. II, John Murray, London
- Brodsky MH, Gambino RJ, Smith JE Jr & Yacoby Y 1972, "The Raman spectrum of amorphous tellurium", Physica Status Solidi B, vol. 52, DOI:10.1002/pssb.2220520229
- Brown TL et al. 2014, Chemistry: The Central Science, 3rd ed., Pearson Australia: Sydney, ISBN 978-1-4425-5460-3
- Burford N, Passmore J & Sanders JCP 1989, "The preparation, structure, and energetics of homopolyatomic cations of groups 16 (the chalcogens) and 17 (the halogens), in Liebman JF & Greenberg A, From atoms to polymers : isoelectronic analogies, VCH: New York, ISBN 978-0-89573-711-3
- Cacace F, de Petris G & Troiani A 2002, "Experimental detection of tetranitrogen", Science, vol. 295, no. 5554, DOI:10.1126/science.1067681
- Cao C et al. 2021, "Understanding periodic and non-periodic chemistry in periodic tables", Frontiers in Chemistry, vol. 8, no. 813, DOI:10.3389/fchem.2020.00813
- Carapella SC 1968, "Arsenic" in Hampel CA (ed.), The Encyclopedia of the Chemical Elements, Reinhold, New York
- Carmalt CJ & Norman NC 1998, 'Arsenic, Antimony and Bismuth: Some General Properties and Aspects of Periodicity', in NC Norman (ed.), Chemistry of Arsenic, Antimony and Bismuth, Blackie Academic & Professional, London, pp. 1–38, ISBN 0-7514-0389-X
- Challoner J 2014, The Elements: The New Guide to the Building Blocks of our Universe, Carlton Publishing Group, ISBN 978-0-233-00436-5
- Chambers E 1743, in "Metal", Cyclopedia: Or an Universal Dictionary of Arts and Sciences (etc.), vol. 2, D Midwinter, London
- Chambers C & Holliday AK 1982, Inorganic Chemistry, Butterworth & Co., London, ISBN 978-0-408-10822-5
- Charlier J-C, Gonze X, Michenaud J-P 1994, First-principles Study of the Stacking Effect on the Electronic Properties of Graphite(s), Carbon, vol. 32, no. 2, pp. 289–99, DOI:10.1016/0008-6223(94)90192-9
- Chemical Abstracts Service 2021, CAS REGISTRY database as of November 2, Case #01271182
- Cherim SM 1971, Chemistry for Laboratory Technicians, Saunders, Philadelphia, ISBN 978-0-7216-2515-7
- Chung DD 1987, "Review of exfoliated graphite", Journal of Materials Science, vol. 22, DOI:10.1007/BF01132008
- Clugston MJ & Flemming R 2000, Advanced Chemistry, Oxford University Press, Oxford, ISBN 978-0-19-914633-8
- Cockell C 2019, The Equations of Life: How Physics Shapes Evolution, Atlantic Books, London, ISBN 978-1-78649-304-0
- Cook CG 1923, Chemistry in Everyday Life: With Laboratory Manual, D Appleton, New York
- Cotton A et al. 1999, Advanced Inorganic Chemistry, 6th ed., Wiley, New York, ISBN 978-0-471-19957-1
- Cousins DM, Davidson MG & García-Vivó D 2013, "Unprecedented participation of a four-coordinate hydrogen atom in the cubane core of lithium and sodium phenolates", Chemical Communications, vol. 49, DOI:10.1039/C3CC47393G
- Cox AN (ed.) 2000, Allen's Astrophysical Quantities, 4th ed., AIP Press, New York, ISBN 978-0-387-98746-0
- Cox PA 1997, The Elements: Their Origins, Abundance, and Distribution, Oxford University Press, Oxford, ISBN 978-0-19-855298-7
- Cox T 2004, Inorganic Chemistry, 2nd ed., BIOS Scientific Publishers, London, ISBN 978-1-85996-289-3
- Crawford FH 1968, Introduction to the Science of Physics, Harcourt, Brace & World, New York
- Crichton R 2012, Biological Inorganic Chemistry: A New Introduction to Molecular Structure and Function, 2nd ed., Elsevier, Amsterdam, ISBN 978-0-444-53783-6
- Cressey D 2010, "Chemists re-define hydrogen bond", Nature newsblog, accessed August 23, 2017
- Criswell B 2007, "Mistake of having students be Mendeleev for just a day", Journal of Chemical Education, vol. 84, no. 7, pp. 1140–1144, DOI:10.1021/ed084p1140
- Dalton L 2019, "Argon reacts with nickel under pressure-cooker conditions", Chemical & Engineering News, accessed November 6, 2019
- Daniel PL & Rapp RA 1976, "Halogen corrosion of metals", in Fontana MG & Staehle RW (eds.), Advances in Corrosion Science and Technology, Springer, Boston, DOI:10.1007/978-1-4615-9062-0_2
- de Clave E 1641, New Philosophical Light of True Principles and Elements of Nature, Olivier Devarennes, Paris, accessed February 24, 2022
- de L'Aunay L 1566, Responce au discours de maistre Iacques Grevin, docteur de Paris, qu'il a escript contre le livre de maistre Loys de l'Aunay, medecin en la Rochelle, touchant la faculté de l'antimoine (Response to the Speech of Master Jacques Grévin,... Which He Wrote Against the Book of Master Loys de L'Aunay,... Touching the Faculty of Antimony), De l'Imprimerie de Barthelemi Berton, La Rochelle
- Desai PD, James HM & Ho CY 1984, "Electrical Resistivity of Aluminum and Manganese", Journal of Physical and Chemical Reference Data, vol. 13, no. 4, DOI:10.1063/1.555725
- Dingle A 2017, The Elements: An Encyclopedic Tour of the Periodic Table, Quad Books, Brighton, ISBN 978-0-85762-505-2
- Donohue J 1982, The Structures of the Elements, Robert E. Krieger, Malabar, Florida, ISBN 978-0-89874-230-5
- Du Y, Ouyang C, Shi S & Lei M 2010, 'Ab Initio Studies on Atomic and Electronic Structures of Black Phosphorus', Journal of Applied Physics, vol. 107, no. 9, pp. 093718–1–4, DOI:10.1063/1.3386509
- Dupasquier A 1844, Traité élémentaire de chimie industrielle, Charles Savy Juene, Lyon.
- Ebbing DD & Gammon SD 2007, General Chemistry, 9th ed., Houghton Miffllin, Boston, ISBN 978-0-618-85748-7
- Edelstein NM & Morrs LR 2009, "Chemistry of the Actinide elements", in Nagy S (ed.), Radiochemistry and Nuclear Chemistry: Volume II, Encyclopedia of Life Support Systems, EOLSS Publishers, Oxford, pp. 118–176, ISBN 978-1-84826-577-6
- Edwards PP 2000, "What, why and when is a metal?", in Hall N (ed.), The New Chemistry, Cambridge University, Cambridge, pp. 85–114, ISBN 978-0-521-45224-3
- Edwards PP et al. 2010, "... a metal conducts and a non-metal doesn’t", Philosophical Transactions of the Royal Society A, 2010, vol, 368, no. 1914, DOI:10.1098/rsta.2009.0282
- Edwards PP & Sienko MJ 1983, "On the occurrence of metallic character in the periodic table of the elements", Journal of Chemical Education, vol. 60, no. 9, DOI:10.1021/ed060p691, PubMed
- Elatresh SF & Bonev SA 2020, "Stability and metallization of solid oxygen at high pressure", Physical Chemistry Chemical Physics, vol. 22, no. 22, DOI:10.1039/C9CP05267D
- Emsley J 1971, The Inorganic Chemistry of the Non-metals, Methuen Educational, London, ISBN 978-0-423-86120-4
- Emsley J 2011, Nature's Building Blocks: An A–Z Guide to the Elements, Oxford University Press, Oxford, ISBN 978-0-19-850341-5
- Encyclopædia Britannica 2021, Periodic table, accessed September 21, 2021
- Errandonea D 2020, "Pressure-induced phase transformations," Crystals, vol. 10, DOI:10.3390/cryst10070595
- Evans RC 1966, An Introduction to Crystal Chemistry, 2nd ed., Cambridge University, Cambridge
- Faraday M 1853, The Subject Matter of a Course of Six Lectures on the Non-metallic Elements, (arranged by John Scoffern), Longman, Brown, Green, and Longmans, London
- Florez et al. 2022, From the gas phase to the solid state: The chemical bonding in the superheavy element flerovium, The Journal of Chemical Physics, vol. 157, 064304, DOI:10.1063/5.0097642
- Fortescue JAC 2012, Environmental Geochemistry: A Holistic Approach, Springer-Verlag, New York, ISBN 978-1-4612-6047-9
- Fraps GS 1913, Principles of Agricultural Chemistry, The Chemical Publishing Company, Easton, PA
- Fraústo da Silva JJR & Williams RJP 2001, The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, 2nd ed., Oxford University Press, Oxford, ISBN 978-0-19-850848-9
- Gaffney J & Marley N 2017, General Chemistry for Engineers, Elsevier, Amsterdam, ISBN 978-0-12-810444-6
- Gargaud M et al. (eds.) 2006, Lectures in Astrobiology, vol. 1, part 1: The Early Earth and Other Cosmic Habitats for Life, Springer, Berlin, ISBN 978-3-540-29005-6
- Glinka N 1958, General chemistry, Sobolev D (trans.), Foreign Languages Publishing House, Moscow
- Godfrin H & Lauter HJ 1995, "Experimental properties of 3He adsorbed on graphite", in Halperin WP (ed.), Progress in Low Temperature Physics, volume 14, Elsevier Science B.V., Amsterdam, ISBN 978-0-08-053993-5
- Godovikov AA & Nenasheva N 2020, Structural-chemical Systematics of Minerals, 3rd ed., Springer, Cham, Switzerland, ISBN 978-3-319-72877-3
- Goodrich BG 1844, A Glance at the Physical Sciences, Bradbury, Soden & Co., Boston
- Government of Canada 2015, Periodic table of the elements, accessed August 30, 2015
- Greenwood NN & Earnshaw A 2002, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, ISBN 978-0-7506-3365-9
- Grochala W 2018, "On the position of helium and neon in the Periodic Table of Elements", Foundations of Chemistry, vol. 20, pp. 191–207, DOI:10.1007/s10698-017-9302-7
- Gusmão R, Sofer Z & Pumera M 2017, "Black phosphorus rediscovered: From bulk material to monolayers", Angewandte Chemie International Edition, vol. 56, no. 28, DOI:10.1002/anie.201610512
- Hampel CA & Hawley GG 1976, Glossary of Chemical Terms, Van Nostrand Reinhold, New York, ISBN 978-0-442-23238-2
- Hanley JJ & Koga KT 2018, "Halogens in terrestrial and cosmic geochemical systems: Abundances, geochemical behaviours, and analytical methods" in The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes: Surface, Crust, and Mantle, Harlov DE & Aranovich L (eds.), Springer, Cham, ISBN 978-3-319-61667-4
- Hare RA & Bache F 1836, Compendium of the Course of Chemical Instruction in the Medical Department of the University of Pennsylvania, 3rd ed., JG Auner, Philadelphia
- Hengeveld R & Fedonkin MA 2007, "Bootstrapping the energy flow in the beginning of life", Acta Biotheoretica, vol. 55, DOI:10.1007/s10441-007-9019-4
- Herman ZS 1999, "The nature of the chemical bond in metals, alloys, and intermetallic compounds, according to Linus Pauling", in Maksić, ZB, Orville-Thomas WJ (eds.), 1999, Pauling's Legacy: Modern Modelling of the Chemical Bond, Elsevier, Amsterdam, DOI:10.1016/S1380-7323(99)80030-2
- Hermann A, Hoffmann R & Ashcroft NW 2013, "Condensed Astatine: Monatomic and metallic", Physical Review Letters, vol. 111, DOI:10.1103/PhysRevLett.111.116404
- Hérold A 2006, "An arrangement of the chemical elements in several classes inside the periodic table according to their common properties", Comptes Rendus Chimie, vol. 9, no. 1, DOI:10.1016/j.crci.2005.10.002
- Herzfeld K 1927, "On atomic properties which make an element a metal", Physical Review, vol. 29, no. 5, DOI:10.1103PhysRev.29.701
- Hill G & Holman J 2000, Chemistry in Context, 5th ed., Nelson Thornes, Cheltenham, ISBN 0-17-448307-4
- Hill G, Holman J & Hulme PG 2017, Chemistry in Context, 7th ed., Oxford University Press, Oxford, ISBN 978-0-19-839618-5
- Holderness A & Berry M 1979, Advanced Level Inorganic Chemistry, 3rd ed., Heinemann Educational Books, London, ISBN 978-0-435-65435-1
- Höll, Kling & Schroll E 2007, "Metallogenesis of germanium—A review", Ore Geology Reviews, vol. 30, nos. 3–4, pp. 145–180, DOI:10.1016/j.oregeorev.2005.07.034
- Homberg W 1708, "Des Essais de Chimie", in Histoire De L'Academie Royale Des Sciences: Avec les Memoires de Mathematique & de Physique, L'Académie, Paris
- Horvath AL 1973, "Critical temperature of elements and the periodic system", Journal of Chemical Education, vol. 50, no. 5, DOI:10.1021/ed050p335
- House JE 2008, Inorganic Chemistry, Elsevier, Amsterdam, ISBN 978-0-12-356786-4
- Housecroft CE & Sharpe AG 2008, Inorganic Chemistry, 3rd ed., Prentice-Hall, Harlow, ISBN 978-0-13-175553-6
- Hurlbut Jr CS 1961, Manual of Mineralogy, 15th ed., John Wiley & Sons, New York
- IUPAC Periodic Table of the Elements, accessed October 11, 2021
- Janas D, Cabrero-Vilatela, A & Bulmer J 2013, "Carbon nanotube wires for high-temperature performance", Carbon, vol. 64, pp. 305–314, DOI:10.1016/j.carbon.2013.07.067
- Jenkins GM & Kawamura K 1976, Polymeric Carbons—Carbon Fibre, Glass and Char, Cambridge University Press, Cambridge, ISBN 978-0-521-20693-8
- Jentzsch AV & Matile S 2015, "Anion transport with halogen bonds", in Metrangolo P & Resnati G (eds.), Halogen Bonding I: Impact on Materials Chemistry and Life Sciences, Springer, Cham, ISBN 978-3-319-14057-5
- Johnson D (ed.) 2007, Metals and Chemical Change, RSC Publishing, Cambridge, ISBN 978-0-85404-665-2
- Johnson RC 1966, Introductory Descriptive Chemistry, WA Benjamin, New York
- Jolly WL 1966, The Chemistry of the Non-metals, Prentice-Hall, Englewood Cliffs, New Jersey
- Jones BW 2010, Pluto: Sentinel of the Outer Solar System, Cambridge University, Cambridge, ISBN 978-0-521-19436-5
- Kaiho T 2017, Iodine Made Simple, CRC Press, e-book, DOI:10.1201/9781315158310
- Keeler J & Wothers P 2013, Chemical Structure and Reactivity: An Integrated Approach, Oxford University Press, Oxford, ISBN 978-0-19-960413-5
- Kendall EA 1811, Pocket encyclopædia, 2nd ed., vol. III, Longman, Hurst, Rees, Orme, and Co., London
- Khan N 2001, An Introduction to Physical Geography, Concept Publishing, New Delhi, ISBN 978-81-7022-898-1
- Kim MG 2000, "Chemical analysis and the domains of reality: Wilhelm Homberg's Essais de chimie, 1702–1709", Studies in History and Philosophy of Science Part A, vol. 31, no. 1, pp. 37–69, DOI:10.1016/S0039-3681(99)00033-3
- King RB 1994, Encyclopedia of Inorganic Chemistry, vol. 3, John Wiley & Sons, New York, ISBN 978-0-471-93620-6
- King RB 1995, Inorganic Chemistry of Main Group Elements, VCH, New York, ISBN 978-1-56081-679-9
- King GB & Caldwell WE 1954, The Fundamentals of College Chemistry, American Book Company, New York
- Kläning UK & Appelman EH 1988, "Protolytic properties of perxenic acid", Inorganic Chemistry, vol. 27, no. 21, DOI:10.1021/ic00294a018
- Klein U 1994, "Origin of the concept of chemical compound", Science in Context, no. 7, vol. 2, pp. 163–204, DOI:10.1017/s0269889700001666
- Kneen WR, Rogers MJW & Simpson P 1972, Chemistry: Facts, Patterns, and Principles, Addison-Wesley, London, ISBN 978-0-201-03779-1
- Knight J 2002, Science of Everyday Things: Real-life chemistry, Gale Group, Detroit, ISBN 9780787656324
- Kosanke et al. 2012, Encyclopedic Dictionary of Pyrotechnics (and Related Subjects), Part 3 – P to Z, Pyrotechnic Reference Series No. 5, Journal of Pyrotechnics, Whitewater, Colorado, ISBN 978-1-889526-21-8
- Koziel JA 2002, "Sampling and sample preparation for indoor air analysis", in Pawliszyn J (ed.), Comprehensive Analytical Chemistry, vol. 37, Elsevier Science B.V., Amsterdam, ISBN 978-0-444-50510-1
- Kubaschewski O 1949, "The change of entropy, volume and binding state of the elements on melting", Transactions of the Faraday Society, vol. 45, DOI:10.1039/TF9494500931
- Labinger JA 2019, "The history (and pre-history) of the discovery and chemistry of the noble gases", in Giunta CJ, Mainz VV & Girolami GS (eds.), 150 Years of the Periodic Table: A Commemorative Symposium, Springer Nature, Cham, Switzerland, ISBN 978-3-030-67910-1
- Lanford OE 1959, Using Chemistry, McGraw-Hill, New York
- Langley RH & Hattori H 2014, 1,001 Practice Problems: Chemistry For Dummies, John Wiley & Sons, Hoboken, NJ, ISBN 978-1-118-54932-2
- Larrañaga MD, Lewis RJ & Lewis RA 2016, Hawley's Condensed Chemical Dictionary, 16th ed., Wiley, Hoboken, New York, ISBN 978-1-118-13515-0
- Leach RB & Ewing GW 1966, Chemistry, Doubleday, New York
- Lee JD 1996, Concise Inorganic Chemistry, 5th ed., Blackwell Science, Oxford, ISBN 978-0-632-05293-6
- Lewis RJ 1993, Hawley's Condensed Chemical Dictionary, 12th ed., Van Nostrand Reinhold, New York, ISBN 978-0-442-01131-4
- Lidin RA 1996, Inorganic Substances Handbook, Begell House, New York, ISBN 978-0-8493-0485-9
- Liptrot GF 1983, Modern Inorganic Chemistry, 4th ed., Bell & Hyman, ISBN 978-0-7135-1357-8
- Los Alamos National Laboratory 2021, Periodic Table of Elements: A Resource for Elementary, Middle School, and High School Students, accessed September 19, 2021
- Luchinskii GP & Trifonov DN 1981, "Some problems of chemical elements classification and the structure of the periodic system", in Uchenie o Periodichnosti. Istoriya i Sovremennoct, (Russian) Nauka, Moscow
- MacKay KM, MacKay RA & Henderson W 2002, Introduction to Modern Inorganic Chemistry, 6th ed., Nelson Thornes, Cheltenham, ISBN 978-0-7487-6420-4
- Mackin M 2014, Study Guide to Accompany Basics for Chemistry, Elsevier Science, Saint Louis, ISBN 978-0-323-14652-4
- Maosheng M 2020, "Noble gases in solid compounds show a rich display of chemistry with enough pressure", Frontiers in Chemistry, vol. 8, DOI:10.3389/fchem.2020.570492
- Massey AG 2000, Main group chemistry, 2nd ed., John Wiley & Sons, Chichester, ISBN 978-0-471-49039-5
- Masterton W, Hurley C & Neth E 2011, Chemistry: Principles and Reactions, 7th ed., Brooks/Cole, Belmont, California, ISBN 978-1-111-42710-8
- Matson M & Orbaek AW 2013, Inorganic Chemistry for Dummies, John Wiley & Sons: Hoboken, ISBN 978-1-118-21794-8
- Matula RA 1979, "Electrical resistivity of copper, gold, palladium, and silver", Journal of Physical and Chemical Reference Data, vol. 8, no. 4, DOI:10.1063/1.555614
- Mazej Z 2020, "Noble-gas chemistry more than half a century after the first report of the noble-gas compound", Molecules, vol. 25, no. 13, DOI:10.3390/molecules25133014, PubMed, قالب:PMC
- McCue JJ 1963, World of Atoms: An Introduction to Physical Science, Ronald Press, New York
- McMillan P 2006, "A glass of carbon dioxide", Nature, vol. 441, DOI:10.1038/441823a
- Messler Jr RW 2011, The Essence of Materials for Engineers, Jones and Bartlett Learning, Sudbury, Massachusetts, ISBN 978-0-7637-7833-0
- Mewes et al. 2019, Copernicium: A relativistic noble liquid, Angewandte Chemie International Edition, vol. 58, pp. 17964–17968, DOI:10.1002/anie.201906966
- Mingos DMP 2019, "The discovery of the elements in the Periodic Table", in Mingos DMP (ed.), The Periodic Table I. Structure and Bonding, Springer Nature, Cham, DOI:10.1007/978-3-030-40025-5
- Moeller T et al. 2012, Chemistry: With Inorganic Qualitative Analysis, Academic Press, New York, ISBN 978-0-12-503350-3
- Möller D 2003, Luft: Chemie, Physik, Biologie, Reinhaltung, Recht, Walter de Gruyter, Berlin, ISBN 978-3-11-016431-2
- Moody B 1991, Comparative Inorganic Chemistry, 3rd ed., Edward Arnold, London, ISBN 978-0-7131-3679-1
- Moore JT 2016, Chemistry for Dummies, 2nd ed., ch. 16, Tracking periodic trends, John Wiley & Sons: Hoboken, ISBN 978-1-119-29728-4
- Morita A 1986, 'Semiconducting Black Phosphorus', Journal of Applied Physics A, vol. 39, no. 4, pp. 227–42, DOI:10.1007/BF00617267
- Morely HF & Muir MM 1892, Watt's Dictionary of Chemistry, vol. 3, Longman's Green, and Co., London
- Moss, TS 1952, Photoconductivity in the Elements, Butterworths Scientific, London
- Nakao Y 1992, "Dissolution of noble metals in halogen–halide–polar organic solvent systems", Journal of the Chemical Society, Chemical Communications, no. 5, DOI:10.1039/C39920000426
- National Center for Biotechnology Information 2021, "PubChem compound summary for CID 402, Hydrogen sulfide", accessed August 31, 2021
- National Institute of Standards and Technology 2013, SRM 4972 – Radon-222 Emanation Standard, accessed August 1, 2021
- Nelson PG 1987, "Important elements", Journal of Chemical Education, vol. 68, no. 9, DOI:10.1021/ed068p732
- Oderberg DS 2007, Real Essentialism, Routledge, New York, ISBN 978-1-134-34885-5
- Ostriker JP & Steinhardt PJ 2001, "The quintessential universe", Scientific American, vol. 284, no. 1, pp. 46–53 PubMed, DOI:10.1038/scientificamerican0101-46 10.1038/scientificamerican0101-46
- Oxtoby DW, Gillis HP & Butler LJ 2015, Principles of Modern Chemistry, 8th ed., Cengage Learning, Boston, ISBN 978-1-305-07911-3
- Parameswaran P et al. 2020, "Phase evolution and characterization of mechanically alloyed hexanary Al16.6Mg16.6Ni16.6Cr16.6Ti16.6Mn16.6 high entropy alloy", Metal Powder Report, vol. 75, no. 4, DOI:10.1016/j.mprp.2019.08.001
- Parish RV 1977, The Metallic Elements, Longman, London, ISBN 978-0-582-44278-8
- Partington JR 1944, A Text-book of Inorganic Chemistry, 5th ed., Macmillan & Co., London
- Petruševski VM & Cvetković J 2018, "On the 'true position' of hydrogen in the Periodic Table", Foundations of Chemistry, vol. 20, pp. 251–260, DOI:10.1007/s10698-018-9306-y
- Phillips CSG & Williams RJP 1965, Inorganic Chemistry, vol. 1, Principles and non-metals, Clarendon Press, Oxford
- Phillips JC 1973, "The chemical structure of solids," in Hannay NB (ed.), Treatise on Solid State Chemistry, vol. 1, Plenum Press, New York, pp. 1–42, ISBN 978-1-4684-2663-2
- Piro NA et al. 2006, "Triple-bond reactivity of diphosphorus molecules", Science, vol. 313, no. 5791, DOI:10.1126/science.1129630, PubMed
- Pitzer K 1975, "Fluorides of radon and elements 118", Journal of the Chemical Society, Chemical Communications, no. 18, DOI:10.1039/C3975000760B
- Porterfield WW 1993, Inorganic chemistry, Academic Press, San Diego, ISBN 978-0-12-562980-5
- Povh B & Rosina M 2017, Scattering and Structures: Essentials and Analogies in Quantum Physics, 2nd ed., Springer, Berlin, DOI:10.1007/978-3-662-54515-7
- Powell P & Timms P 1974, The Chemistry of the Non-Metals, Chapman and Hall, London, ISBN 978-0-412-12200-2
- Puddephatt RJ & Monaghan PK 1989, The Periodic Table of the Elements, 2nd ed., Clarendon Press, Oxford, ISBN 978-0-19-855516-2
- Rahm M, Zeng T & Hoffmann R 2019, "Electronegativity seen as the ground-state average valence electron binding energy", Journal of the American Chemical Society, vol. 141, no. 1, pp. 342−351, DOI:10.1021/jacs.8b10246
- Rao KY 2002, Structural Chemistry of Glasses, Elsevier, Oxford, ISBN 978-0-08-043958-7
- Rao CNR & Ganguly PA 1986, "New criterion for the metallicity of elements", Solid State Communications, vol. 57, no. 1, pp. 5–6, DOI:10.1016/0038-1098(86)90659-9
- Rayner-Canham G 2018, "Organizing the transition metals", in Scerri E & Restrepo G, Mendeleev to Oganesson: A multidisciplinary perspective on the periodic table, Oxford University, New York, ISBN 978-0-190-668532
- Rayner-Canham G 2020, The Periodic Table: Past, Present and Future, World Scientific, New Jersey, ISBN 978-981-121-850-7
- Regnault MV 1853, Elements of Chemistry, vol. 1, 2nd ed., Clark & Hesser, Philadelphia
- Reilly C 2002, Metal Contamination of Food, Blackwell Science, Oxford, ISBN 978-0-632-05927-0
- Remy H 1956, Treatise on Inorganic Chemistry, Anderson JS (trans.), Kleinberg J (ed.), vol. II, Elsevier, Amsterdam
- Renouf E 1901, "Lehrbuch der anorganischen Chemie", Science, vol. 13, no. 320, DOI:10.1126/science.13.320.268
- Restrepo G, Llanos EJ & Mesa H 2006, "Topological space of the chemical elements and its properties", Journal of Mathematical Chemistry, vol. 39, DOI:10.1007/s10910-005-9041-1
- Ritter SK 2011, "The case of the missing xenon", Chemical & Engineering News, vol. 89, no. 9, ISSN 0009-2347
- Rochow EG 1966, The Metalloids, DC Heath and Company, Boston
- Rochow EG 1973, "Silicon", in Bailar JC et al. (eds.), Comprehensive Inorganic Chemistry, vol. 1, Pergamon Press, Oxford, ISBN 978-0-08-015655-2
- Rodgers GE 2012, Descriptive Inorganic, Coordination, and Solid State Chemistry, 3rd ed., Brooks/Cole, Belmont, California, ISBN 978-0-8400-6846-0
- Royal Society of Chemistry 2021, Periodic Table: Non-metal, accessed September 3, 2021
- Rudolph J 1973, Chemistry for the Modern Mind, Macmillan, New York
- Russell AM & Lee KL 2005, Structure-Property Relations in Nonferrous Metals, Wiley-Interscience, New York, ISBN 0-471-64952-X
- Salinas JT 2019 Exploring Physical Science in the Laboratory, Moreton Publishing, Englewood, Colorado, ISBN 978-1-61731-753-8
- Salzberg HW 1991, From Caveman to Chemist: Circumstances and Achievements, American Chemical Society, Washington, DC, ISBN 0-8412-1786-6
- Sanderson RT 1957, "An electronic distinction between metals and nonmetals", Journal of Chemical Education, vol. 34, no. 5, DOI:10.1021/ed034p229
- Sanderson RT 1967, Inorganic Chemistry, Reinhold, New York
- Scerri E (ed.) 2013, 30-Second Elements: The 50 Most Significant Elements, Each Explained In Half a Minute, Ivy Press, London, ISBN 978-1-84831-616-4
- Schaefer JC 1968, "Boron" in Hampel CA (ed.), The Encyclopedia of the Chemical Elements, Reinhold, New York
- Schlager N & Lauer J (eds.) 2000, Science and Its Times: 1700–1799, volume 4 of Science and its times: Understanding the social significance of scientific discovery, Gale Group, ISBN 978-0-7876-3932-7
- Schmedt auf der Günne J, Mangstl M & Kraus F 2012, "Occurrence of difluorine F2 in nature—In situ proof and quantification by NMR spectroscopy", Angewandte Chemie International Edition, vol. 51, no. 31, DOI:10.1002/anie.201203515
- Scott D 2014, Around the World in 18 Elements, Royal Society of Chemistry, e-book, ISBN 978-1-78262-509-4
- Scott EC & Kanda FA 1962, The Nature of Atoms and Molecules: A General Chemistry, Harper & Row, New York
- Seese WS & Daub GH 1985, Basic Chemistry, 4th ed., Prentice-Hall, Englewood Cliffs, NJ, ISBN 978-0-13-057811-2
- Segal BG 1989, Chemistry: Experiment and Theory, 2nd ed., John Wiley & Sons, New York, ISBN 0-471-84929-4
- Shanabrook BV, Lannin JS & Hisatsune IC 1981, "Inelastic light scattering in a onefold-coordinated amorphous semiconductor", Physical Review Letters, vol. 46, no. 2, 12 January, DOI:10.1103/PhysRevLett.46.130
- Shang et al. 2021, "Ultrahard bulk amorphous carbon from collapsed fullerene", Nature, vol. 599, pp. 599–604, DOI:10.1038/s41586-021-03882-9
- Sherwin E & Weston GJ 1966, Chemistry of the Non-metallic Elements, Pergamon Press, Oxford
- Shiell et al. 2021, "Bulk crystalline 4H-silicon through a metastable allotropic transition", Physical Review Letters, vol. 26, p 215701, DOI:10.1103/PhysRevLett.126.215701
- Shkol’nikov EV 2010, "Thermodynamic characterization of the amphoterism of oxides M2O3 (M = As, Sb, Bi) and their hydrates in aqueous media, Russian Journal of Applied Chemistry, vol. 83, no. 12, pp. 2121–2127, DOI:10.1134/S1070427210120104
- Sidorov TA 1960, "The connection between structural oxides and their tendency to glass formation", Glass and Ceramics, vol. 17, no. 11, DOI:10.1007BF00670116
- Siekierski S & Burgess J 2002, Concise Chemistry of the Elements, Horwood Press, Chichester, ISBN 978-1-898563-71-6
- Smith A & Dwyer C 1991, Key Chemistry: Investigating Chemistry in the Contemporary World: Book 1: Materials and Everyday Life, Melbourne University Press, Carlton, Victoria, ISBN 978-0-522-84450-4
- Smits et al. 2020, Oganesson: A noble gas element that is neither noble nor a gas, Angewandte Chemie International Edition, vol. 59, pp. 23636–23640, DOI:10.1002/anie.202011976
- Stein L 1969, "Oxidized radon in halogen fluoride solutions", Journal of the American Chemical Society, vol. 19, no. 19, DOI:10.1021/ja01047a042
- Stein L 1983, "The chemistry of radon", Radiochimica Acta, vol. 32, DOI:10.1524/ract.1983.32.13.163
- Stellman JM (ed.) 1998, Encyclopaedia of Occupational Health and Safety, vol. 4, 4th ed., International Labour Office, Geneva, ISBN 978-92-2-109817-1
- Steudel R 1977, Chemistry of the Non-metals: With an Introduction to atomic Structure and Chemical Bonding, Walter de Gruyter, Berlin, ISBN 978-3-11-004882-7
- Steudel R & Eckert B 2003, "Solid sulfur allotropes", in Steudel R (ed.), Elemental Sulfur and Sulfur-rich Compounds I, Springer-Verlag, Berlin, ISBN 978-3-540-40191-9
- Steudel R 2020, Chemistry of the Non-metals: Syntheses - Structures - Bonding - Applications, in collaboration with D Scheschkewitz, Berlin, Walter de Gruyter, DOI:10.1515/9783110578065
- Still B 2016 The secret life of the periodic table, Cassell, London, ISBN 978-1-84403-885-5
- Stott RWA 1956, Companion to Physical and Inorganic Chemistry, Longmans, Green and Co, London
- Stuke J 1974, 'Optical and electrical properties of selenium', in RA Zingaro & WC Cooper (eds), Selenium, Van Nostrand Reinhold, New York, pp. 174
- Strathern P 2000, Mendeleyev's dream: The Quest for the Elements, Hamish Hamilton, London, ISBN 978-0-8412-1786-7
- Su et al. 2020, "Advances in photonics of recently developed Xenes", Nanophotonics, vol. 9, no. 7, DOI:10.1515/nanoph-2019-0561
- Suresh CH & Koga NA 2001, "A consistent approach toward atomic radii”, Journal of Physical Chemistry A, vol. 105, no. 24. DOI:10.1021/jp010432b
- Tang et al. 2021, "Synthesis of paracrystalline diamond", Nature, vol. 599, pp. 605–610, DOI:10.1038/s41586-021-04122-w
- Taniguchi M, Suga S, Seki M, Sakamoto H, Kanzaki H, Akahama Y, Endo S, Terada S & Narita S 1984, 'Core-exciton induced resonant photoemission in the covalent semiconductor black phosphorus', Solid State Communications, vo1. 49, no. 9, pp. 867–7, DOI:10.1016/0038-1098(84)90441-1
- Taylor MD 1960, First Principles of Chemistry, Van Nostrand, Princeton
- The Chemical News and Journal of Physical Science 1864, "Notices of books: Manual of the Metalloids", vol. 9, p. 22
- The Chemical News and Journal of Physical Science 1897, "Notices of books: A Manual of Chemistry, Theoretical and Practical", by WA Tilden", vol. 75, pp. 188–189
- Thornton BF & Burdette SC 2010, "Finding eka-iodine: Discovery priority in modern times", Bulletin for the history of chemistry, vol. 35, no. 2, accessed September 14, 2021
- Tregarthen L 2003, Preliminary Chemistry, Macmillan Education: Melbourne, ISBN 978-0-7329-9011-4
- Trenberth KE & Smith L 2005, "The mass of the atmosphere: A constraint on global analyses", Journal of Climate, vol. 18, no. 6, DOI:10.1175/JCLI-3299.1
- Tshitoyan et al. 2019, "Unsupervised word embeddings capture latent knowledge from materials science literature", Nature, vol. 571, DOI:10.1038/s41586-019-1335-8
- Tyler PM 1948, From the Ground Up: Facts and Figures of the Mineral Industries of the United States, McGraw-Hill, New York
- Vassilakis AA, Kalemos A & Mavridis A 2014, "Accurate first principles calculations on chlorine fluoride ClF and its ions ClF±", Theoretical Chemistry Accounts, vol. 133, no. 1436, DOI:10.1007/s00214-013-1436-7
- Vernon R 2013, "Which elements are metalloids?", Journal of Chemical Education, vol. 90, no. 12, 1703‒1707, DOI:10.1021/ed3008457
- Vernon R 2020, "Organising the metals and nonmetals", Foundations of Chemistry, vol. 22, DOI:10.1007/s10698-020-09356-6 (open access)
- Wächtershäuser G 2014, "From chemical invariance to genetic variability", in Weigand W and Schollhammer P (eds.), Bioinspired Catalysis: Metal Sulfur Complexes, Wiley-VCH, Weinheim, DOI:10.1002/9783527664160.ch1
- Wakeman TH 1899, "Free thought—Past, present and future", Free Thought Magazine, vol. 17
- Wasewar KL 2021, "Intensifying approaches for removal of selenium", in Devi et al. (eds), Selenium contamination in water, John Wiley & Sons, Hoboken, pp. 319–355, ISBN 978-1-119-69354-3
- Weeks ME 1945, Discovery of the Elements, 5th ed., Journal of Chemical Education, Easton, Pennsylvania
- Welcher SH 2001, High marks: Regents Chemistry Made Easy, 2nd ed., High Marks Made Easy, New York, ISBN 978-0-9714662-4-1
- Wells AF 1984, Structural Inorganic Chemistry, 5th ed., Clarendon Press, Oxford, ISBN 978-0-19-855370-0
- White JH 1962, Inorganic Chemistry: Advanced and Scholarship Levels, University of London Press, London
- Wiberg N 2001, Inorganic Chemistry, Academic Press, San Diego, ISBN 978-0-12-352651-9
- Williams RPJ 2007, "Life, the environment and our ecosystem", Journal of Inorganic Biochemistry, vol. 101, nos. 11–12, DOI:10.1016/j.jinorgbio.2007.07.006
- Woodward et al. 1999, "The electronic structure of metal oxides", In Fierro JLG (ed.), Metal Oxides: Chemistry and Applications, CRC Press, Boca Raton, ISBN 1-4200-2812-X
- Wulfsberg G 1987, Principles of Descriptive Chemistry, Brooks/Cole, Belmont CA, ISBN 978-0-534-07494-4
- Wulfsberg G 2000, Inorganic Chemistry, University Science Books, Sausalito, California, ISBN 978-1-891389-01-6
- Yamaguchi M & Shirai Y 1996, "Defect structures", in Stoloff NS & Sikka VK (eds.), Physical Metallurgy and Processing of Intermetallic Compounds, Chapman & Hall, New York, ISBN 978-1-4613-1215-4
- Yang J 2004, 'Theory of thermal conductivity', in Tritt TM (ed.), Thermal Conductivity: Theory, Properties, and Applications, Kluwer Academic/Plenum Publishers, New York, pp. 1–20, ISBN 978-0-306-48327-1,
- Yoder CH, Suydam FH & Snavely FA 1975, Chemistry, 2nd ed, Harcourt Brace Jovanovich, New York, ISBN 978-0-15-506470-6
- Young JA 2006, "Iodine", Journal of Chemical Education, vol. 83, no. 9, DOI:10.1021/ed083p1285
- Young et al. 2018, General Chemistry: Atoms First, Cengage Learning: Boston, ISBN 978-1-337-61229-6
- Yousuf M 1998, "Diamond anvil cells in high-pressure studies of semiconductors", in Suski T & Paul W (eds.), High Pressure in Semiconductor Physics II, Semiconductors and Semimetals, vol. 55, Academic Press, San Diego, ISBN 978-0-08-086453-2
- Zhao J, Tu Z & Chan SH 2021, Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): A review, Journal of Power Sources, vol. 488, #229434, DOI:10.1016/j.jpowsour.2020.229434
- Zhao Z, Zhang H, Kim D. et al. 2017, "Properties of the exotic metastable ST12 germanium allotrope", Nature Communications, vol. 8, article no. 13909, DOI:10.1038/ncomms13909, قالب:Pmid, قالب:Pmc
- Zhigal'skii GP & Jones BK 2003, The Physical Properties of Thin Metal Films, Taylor & Francis, London, ISBN 978-0-415-28390-8
- Zhu et al. 2014, "Reactions of xenon with iron and nickel are predicted in the Earth's inner core", Nature Chemistry, vol. 6, DOI:10.1038/nchem.1925, قالب:Pmid
- Zumdahl SS & DeCoste DJ 2010, Introductory Chemistry: A Foundation, 7th ed., Cengage Learning, Mason, Ohio, ISBN 978-1-111-29601-8
المصادر
هيام بيرقدار. "اللامعدن". الموسوعة العربية.
- ويكيبيديا الإنجليزية.
وصلات خارجية
 Media related to Nonmetals at Wikimedia Commons
Media related to Nonmetals at Wikimedia Commons







