ثالث أكسيد الكروم
| |

| |
| الأسماء | |
|---|---|
| اسم أيوپاك
Chromium trioxide
| |
| أسماء أخرى
Chromic anhydride, Chromium(VI) oxide, Chromic acid (misnomer)
| |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| رقم RTECS |
|
| UNII | |
| UN number | 1463 |
| |
| |
| الخصائص | |
| الصيغة الجزيئية | CrO3 |
| كتلة مولية | 99.98 g mol-1 |
| المظهر | Dark red granular solid, deliquescent |
| الرائحة | Odorless |
| الكثافة | 2.7 g/cm3 (20 °C)[1] |
| نقطة الانصهار | |
| نقطة الغليان | |
| قابلية الذوبان في الماء | 164.8 g/100 mL (0 °C) 169 g/100 mL (25 °C)[1] 172.6 g/100 mL (40 °C) 198.1 g/100 mL (100 °C)[2] |
| قابلية الذوبان | Soluble in H2SO4, HNO3, (C2H5)2O, CH3COOH, acetone |
| القابلية المغناطيسية | +40·10−6 cm3/mol[1] |
| الكيمياء الحرارية | |
| الإنتالپية المعيارية للتشكل ΔfH |
−589.3 kJ/mol[3] |
| Standard molar entropy S |
73.2 J/mol·K[4] |
| المخاطر | |
| صفحة بيانات السلامة | ICSC 1194 |
| ن.م.ع. مخطط تصويري |      [5] [5]
|
| ن.م.ع. كلمة الاشارة | Danger |
| H271, H301+H311, H314, H317, H330, H334, H335, H340, H350, H361f, H372, H410[5] | |
| P210, P260, P280, P303+P361+P353, P304+P340+P310, P305+P351+P338[5] | |
| NFPA 704 (معيـَّن النار) | |
| الجرعة أو التركيز القاتل (LD, LC): | |
LD50 (الجرعة الوسطى)
|
80 mg/kg (rats, oral)[6] |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
ثالث أكسيد الكروم Chromium trioxide (ويُعرف أيضاً بإسم أكسيد الكروم السداسي أو أنهيدريد الكروميك) هو مركب غير عضوي بصيغة CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.[6] This compound is a dark-purple solid under anhydrous conditions, bright orange when wet and which dissolves in water concomitant with hydrolysis. ملايين الكيلوگرامات تُنتج منه سنوياً، أساساً للطلاء الكهربائي.[7] ثالث أكسيد الكروم هو مؤكسد قوي ومسرطن.
الانتاج والبنية والتفاعلات الأساسية
Chromium trioxide is generated by treating sodium chromate or the corresponding sodium dichromate with sulfuric acid:[6]
- H2SO4 + Na2Cr2O7 → 2 CrO3 + Na2SO4 + H2O
Approximately 100,000 tonnes are produced annually by this or similar routes.[7]
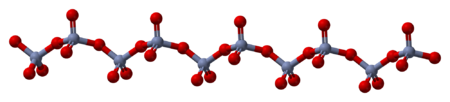
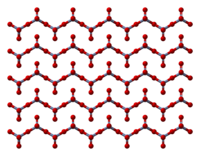
The solid consists of chains of tetrahedrally coordinated chromium atoms that share vertices. Each chromium center therefore shares two oxygen centers with neighbors. Two oxygen atoms are not shared, giving an overall stoichiometry of 1:3.[8][9]
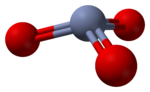
The structure of monomeric CrO3 has been calculated using density functional theory, and is predicted to be pyramidal (point group C3v) rather than planar (point group D3h).[10]
Chromium trioxide decomposes above 197 °C, liberating oxygen and eventually giving Cr2O3:
- 4 CrO3 → 2 Cr2O3 + 3 O2
It is used in organic synthesis as an oxidant, often as a solution in acetic acid,[8] or acetone in the case of the Jones oxidation. In these oxidations, the Cr(VI) converts primary alcohols to the corresponding carboxylic acids and secondary alcohols to ketones. The reactions are shown below:
- Primary alcohols to carboxylic acids
- 4 CrO3 + 3 RCH2OH + 12 H+ → 3 RCOOH + 4 Cr3+ + 9 H2O
- Secondary alcohols to ketones
- 2 CrO3 + 3 R2CHOH + 6 H+ → 3 R2C=O + 2 Cr3+ + 6 H2O
التطبيقات
Chromium trioxide is mainly used in chrome plating. It is typically employed with additives that affect the plating process but do not react with the trioxide. The trioxide reacts with cadmium, zinc, and other metals to generate passivating chromate films that resist corrosion. It is also used in the production of synthetic rubies. Chromic acid solution is also used in applying types of anodic coating to aluminium, which are primarily used in aerospace applications. On the International Space Station, it is used to control bacteria growth in the wastewater storage tank. A chromic acid/phosphoric acid solution is also the preferred stripping agent of anodic coatings of all types.
السلامة
Chromium trioxide is highly toxic, corrosive, and carcinogenic.[11] It is the main example of hexavalent chromium, an environmental hazard.[12]The related chromium(III) derivatives are not particularly dangerous; thus, reductants are used to destroy chromium(VI) samples.
Chromium trioxide, being a powerful oxidizer, will ignite organic materials such as alcohols on contact.
الصور
المراجع
- ^ أ ب ت قالب:CRC90
- ^ Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). D. Van Nostrand Company. p. 250.
- ^ Pradyot, Patnaik (2003). Handbook of Inorganic Chemicals. The McGraw-Hill Companies, Inc. ISBN 0-07-049439-8.
- ^ "chromium(VI) oxide". chemister.ru.
- ^ أ ب ت Sigma-Aldrich Co., Chromium(VI) oxide. Retrieved on 2021-11-22.
- ^ أ ب ت ث "Chromium trioxide". chemicalland21.com. AroKor Holdings Inc. Retrieved 2014-06-15.
- ^ أ ب Anger, G.; Halstenberg, J.; Hochgeschwender, K.; Scherhag, C.; Korallus, U.; Knopf, H.; Schmidt, P.; Ohlinger, M. (2000). "Chromium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a07_067. ISBN 3527306730.
- ^ أ ب قالب:Cotton&Wilkinson6th
- ^ Stephens, J. S.; Cruickshank, D. W. J. (1970). "The crystal structure of (CrO3)∞". Acta Crystallographica Section B. 26 (3): 222. doi:10.1107/S0567740870002182.
- ^ Zhai, H. J.; Li, S.; Dixon, D. A.; Wang, L. S. (2008). "Probing the Electronic and Structural Properties of Chromium Oxide Clusters (CrO 3)−n and (CrO3)n (n = 1–5): Photoelectron Spectroscopy and Density Functional Calculations". Journal of the American Chemical Society. 130 (15): 5167–77. doi:10.1021/ja077984d. PMID 18327905.
- ^ "Chromium Trioxide (MSDS)". J. T. Baker. Archived from the original on 2015-01-12. Retrieved 2007-09-13.
- ^ The environmental impact of hexavalent chromium inspired the 2000 biographical Hollywood movie Erin Brockovich.