مندليڤيوم
| Mendelevium في الجدول الدوري | ||||||
|---|---|---|---|---|---|---|
| ||||||
| الرقم الذري (Z) | 101 | |||||
| المجموعة | n/a | |||||
| الدورة | period 7 | |||||
| المستوى الفرعي | f-block | |||||
| التوزيع الإلكتروني | [Rn] 5f13 7s2 | |||||
| الإلكترونات بالغلاف | 2, 8, 18, 32, 31, 8, 2 | |||||
| الخصائص الطبيعية | ||||||
| الطور at د.ح.ض.ق | solid (متوقع) | |||||
| نقطة الانصهار | 1100 K (827 °س، 1521 °F) (predicted) | |||||
| الكثافة (بالقرب من د.ح.غ.) | 10.3(7) ج/سم³ (متوقع)[1][أ] | |||||
| الخصائص الذرية | ||||||
| الكهرسلبية | مقياس پاولنگ: 1.3 | |||||
| طاقات التأين |
| |||||
| خصائص أخرى | ||||||
| التواجد الطبيعي | synthetic | |||||
| البنية البلورية | face-centered cubic (fcc) (متوقع)[1] | |||||
| رقم كاس | 7440-11-1 | |||||
| التاريخ | ||||||
| التسمية | after دميتري مندليڤ | |||||
| الاكتشاف | مختبر لورنس بركلي الوطني (1955) | |||||
| نظائر الmendelevium | ||||||
| قالب:جدول نظائر mendelevium غير موجود | ||||||
مندليڤيوم ( Mendelevium ، ويعرف أيضا أنيلونيوم) هو أحد العناصر الكيميائية الموجودة في الجدول الدوري وله الرمز Md (سابقا Mv) ورقم ذري 101. وهو عنصر فلزي نشيط إشعاعيا بعد اليورانيوم كما انه من الأكتينيدات. ويتم تصنيعه بقذف الأينشتاينيوم بجسيمات ألفا وتم تسميته على شرف العالم دميترى مندليڤ.
تاريخ المندليفيوم
في 30 أبريل 1955، أُعلِن عن إكتشاف العنصر 101 المصطنع، المندليفيوم. وقد تم انتاج النظير Md-256 (له عمر نصف 76 دقيقة) عندما تم قذف أينشتاينيوم-253 بجسيمات ألفا (نواة الهليوم) في معمل بركلي للإشعاع بإستخدام 60-بوصة سيكلوترون (تم الحصول على Md-256 ذرة واحدة لكل مرة). وكان العنصر 101 هو تاسع عنصر من عناصر ما بعد اليورانيوم يتم تصنيعه.

الاكتشاف كان على يد فريق من الكيميائيين بقيادة الحاصل على جائزة نوبل گلن سيبورگ وعضوية ألبرت گيورسو، برنارد هارڤي، گرگ شوپن وس. ج. طومپسون. النظير الناتج كان مشعاً وله عمر نصف يتراوح بين نصف ساعة وعدة ساعات، لذلك فإن هذا العنصر لا يمكن أن يتواجد في الطبيعة، إذ يضمحل بسرعة إلى عناصر أخف. العنصر سُمي مندليڤيوم تكريماً للكيميائي الروسي دميتري مندليڤ الذي عمل على الجدول الدوري للعناصر.
الصفات المميزة
الطبيعية
أظهرت الأبحاث أن المندليفيوم له حالة تأكسد متوسطة الثبات ثنائية موجبة (II), وذلك بالإضافة لحالة التأكسد الثلاثية (III) (لعناصر الأكتينيدات). Md-256 تم إستخدامه لمعرفة الخصائص الكيميائية للعنصر في محلوله المائي. ولا توجد إستخدامات اخرى للمندليفيوم, ولم يتم إنتاج إلا كميات ضئيلة منه.
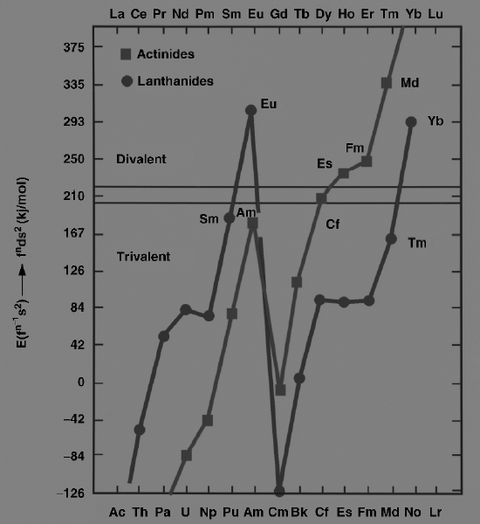
The lanthanides and actinides, in the metallic state, can exist as either divalent (such as europium and ytterbium) or trivalent (most other lanthanides) metals. The former have fns2 configurations, whereas the latter have fn−1d1s2 configurations. In 1975, Johansson and Rosengren examined the measured and predicted values for the cohesive energies (enthalpies of crystallization) of the metallic lanthanides and actinides, both as divalent and trivalent metals.[4][5] The conclusion was that the increased binding energy of the [Rn]5f126d17s2 configuration over the [Rn]5f137s2 configuration for mendelevium was not enough to compensate for the energy needed to promote one 5f electron to 6d, as is true also for the very late actinides: thus einsteinium, fermium, mendelevium, and nobelium were expected to be divalent metals.[4] The increasing predominance of the divalent state well before the actinide series concludes is attributed to the relativistic stabilization of the 5f electrons, which increases with increasing atomic number.[6] Thermochromatographic studies with trace quantities of mendelevium by Zvara and Hübener from 1976 to 1982 confirmed this prediction.[7] In 1990, Haire and Gibson estimated mendelevium metal to have an enthalpy of sublimation between 134 and 142 kJ/mol.[7] Divalent mendelevium metal should have a metallic radius of around 194±10 pm.[7] Like the other divalent late actinides (except the once again trivalent lawrencium), metallic mendelevium should assume a face-centered cubic crystal structure.[1] Mendelevium's melting point has been estimated at 827 °C, the same value as that predicted for the neighboring element nobelium.[8] Its density is predicted to be around 10.3±0.7 g/cm3.[1]
الكيميائية
The chemistry of mendelevium is mostly known only in solution, in which it can take on the +3 or +2 oxidation states. The +1 state has also been reported, but has not yet been confirmed.[9]
Before mendelevium's discovery, Seaborg and Katz predicted that it should be predominantly trivalent in aqueous solution and hence should behave similarly to other tripositive lanthanides and actinides. After the synthesis of mendelevium in 1955, these predictions were confirmed, first in the observation at its discovery that it eluted just after fermium in the trivalent actinide elution sequence from a cation-exchange column of resin, and later the 1967 observation that mendelevium could form insoluble hydroxides and fluorides that coprecipitated with trivalent lanthanide salts.[9] Cation-exchange and solvent extraction studies led to the conclusion that mendelevium was a trivalent actinide with an ionic radius somewhat smaller than that of the previous actinide, fermium.[9] Mendelevium can form coordination complexes with 1,2-cyclohexanedinitrilotetraacetic acid (DCTA).[9]
In reducing conditions, mendelevium(III) can be easily reduced to mendelevium(II), which is stable in aqueous solution.[9] The standard reduction potential of the E°(Md3+→Md2+) couple was variously estimated in 1967 as −0.10 V or −0.20 V:[9] later 2013 experiments established the value as −0.16±0.05 V.[10] In comparison, E°(Md3+→Md0) should be around −1.74 V, and E°(Md2+→Md0) should be around −2.5 V.[9] Mendelevium(II)'s elution behavior has been compared with that of strontium(II) and europium(II).[9]
In 1973, mendelevium(I) was reported to have been produced by Russian scientists, who obtained it by reducing higher oxidation states of mendelevium with samarium(II). It was found to be stable in neutral water–ethanol solution and be homologous to caesium(I). However, later experiments found no evidence for mendelevium(I) and found that mendelevium behaved like divalent elements when reduced, not like the monovalent alkali metals.[9] Nevertheless, the Russian team conducted further studies on the thermodynamics of cocrystallizing mendelevium with alkali metal chlorides, and concluded that mendelevium(I) had formed and could form mixed crystals with divalent elements, thus cocrystallizing with them. The status of the +1 oxidation state is still tentative.[9]
The electrode potential E°(Md4+→Md3+) was predicted in 1975 to be +5.4 V; 1967 experiments with the strong oxidizing agent sodium bismuthate were unable to oxidize mendelevium(III) to mendelevium(IV).[9]
الذرية
A mendelevium atom has 101 electrons, of which at least three (and perhaps four) can act as valence electrons. They are expected to be arranged in the configuration [Rn]5f137s2 (ground state term symbol 2F7/2), although experimental verification of this electron configuration had not yet been made as of 2006.[11] In forming compounds, three valence electrons may be lost, leaving behind a [Rn]5f12 core: this conforms to the trend set by the other actinides with their [Rn] 5fn electron configurations in the tripositive state. The first ionization potential of mendelevium was measured to be at most (6.58 ± 0.07) eV in 1974, based on the assumption that the 7s electrons would ionize before the 5f ones;[12] this value has since not yet been refined further due to mendelevium's scarcity and high radioactivity.[13] The ionic radius of hexacoordinate Md3+ had been preliminarily estimated in 1978 to be around 91.2 pm;[9] 1988 calculations based on the logarithmic trend between distribution coefficients and ionic radius produced a value of 89.6 pm, as well as an enthalpy of hydration of −3654±12 kJ/mol.[9] Md2+ should have an ionic radius of 115 pm and hydration enthalpy −1413 kJ/mol; Md+ should have ionic radius 117 pm.[9]
النظائر
تم التعرف على 15 نظير مشع للمندليڤيوم، وأكثرهم ثباتا Md-258 وله عمر نصف 51.5 يوم, Md-260 وله عمر نصف 31.8 يوم, Md-257 وله عمر نصف 5.52 ساعة. وباقى النظائر الإشعاعية لها عمر نصف أقل من 97 دقيقة, ومعظمها لها عمر نصف أقل من 5 دقائق. كما أن هذا العنصر له حالة رجوع واحدة, 258m-Md (عمر نصف 57 دقيقة). تتراوح نظائر المندليفيوم في الوزن الذري من 245.091 وحدة كتل ذرية (Md-245) إلى 260.104 وحدة كتل ذرية (Md-260).
الانتاج والعزل
The lightest isotopes (244Md to 247Md) are mostly produced through bombardment of bismuth targets with heavy argon ions, while slightly heavier ones (248Md to 253Md) are produced by bombarding plutonium and americium targets with ions of carbon and nitrogen. The most important and most stable isotopes are in the range from 254Md to 258Md and are produced through bombardment of einsteinium with alpha particles: einsteinium-253, -254, and -255 can all be used. 259Md is produced as a daughter of 259No, and 260Md can be produced in a transfer reaction between einsteinium-254 and oxygen-18.[14] Typically, the most commonly used isotope 256Md is produced by bombarding either einsteinium-253 or -254 with alpha particles: einsteinium-254 is preferred when available because it has a longer half-life and therefore can be used as a target for longer.[14] Using available microgram quantities of einsteinium, femtogram quantities of mendelevium-256 may be produced.[14]
The recoil momentum of the produced mendelevium-256 atoms is used to bring them physically far away from the einsteinium target from which they are produced, bringing them onto a thin foil of metal (usually beryllium, aluminium, platinum, or gold) just behind the target in a vacuum.[15] This eliminates the need for immediate chemical separation, which is both costly and prevents reusing of the expensive einsteinium target.[15] The mendelevium atoms are then trapped in a gas atmosphere (frequently helium), and a gas jet from a small opening in the reaction chamber carries the mendelevium along.[15] Using a long capillary tube, and including potassium chloride aerosols in the helium gas, the mendelevium atoms can be transported over tens of meters to be chemically analyzed and have their quantity determined.[16][15] The mendelevium can then be separated from the foil material and other fission products by applying acid to the foil and then coprecipitating the mendelevium with lanthanum fluoride, then using a cation-exchange resin column with a 10% ethanol solution saturated with hydrochloric acid, acting as an eluant. However, if the foil is made of gold and thin enough, it is enough to simply dissolve the gold in aqua regia before separating the trivalent actinides from the gold using anion-exchange chromatography, the eluant being 6 M hydrochloric acid.[15]
Mendelevium can finally be separated from the other trivalent actinides using selective elution from a cation-exchange resin column, the eluant being ammonia α-HIB.[15] Using the gas-jet method often renders the first two steps unnecessary.[15] The above procedure is the most commonly used one for the separation of transeinsteinium elements.[15]
Another possible way to separate the trivalent actinides is via solvent extraction chromatography using bis-(2-ethylhexyl) phosphoric acid (abbreviated as HDEHP) as the stationary organic phase and nitric acid as the mobile aqueous phase. The actinide elution sequence is reversed from that of the cation-exchange resin column, so that the heavier actinides elute later. The mendelevium separated by this method has the advantage of being free of organic complexing agent compared to the resin column; the disadvantage is that mendelevium then elutes very late in the elution sequence, after fermium.[16][15]
Another method to isolate mendelevium exploits the distinct elution properties of Md2+ from those of Es3+ and Fm3+. The initial steps are the same as above, and employs HDEHP for extraction chromatography, but coprecipitates the mendelevium with terbium fluoride instead of lanthanum fluoride. Then, 50 mg of chromium is added to the mendelevium to reduce it to the +2 state in 0.1 M hydrochloric acid with zinc or mercury.[15] The solvent extraction then proceeds, and while the trivalent and tetravalent lanthanides and actinides remain on the column, mendelevium(II) does not and stays in the hydrochloric acid. It is then reoxidized to the +3 state using hydrogen peroxide and then isolated by selective elution with 2 M hydrochloric acid (to remove impurities, including chromium) and finally 6 M hydrochloric acid (to remove the mendelevium).[15] It is also possible to use a column of cationite and zinc amalgam, using 1 M hydrochloric acid as an eluant, reducing Md(III) to Md(II) where it behaves like the alkaline earth metals.[15] Thermochromatographic chemical isolation could be achieved using the volatile mendelevium hexafluoroacetylacetonate: the analogous fermium compound is also known and is also volatile.[15]
السمية
Though few people come in contact with mendelevium, the International Commission on Radiological Protection has set annual exposure limits for the most stable isotope. For mendelevium-258, the ingestion limit was set at 9×105 becquerels (1 Bq = 1 decay per second). Given the half-life of this isotope, this is only 2.48 ng (nanograms). The inhalation limit is at 6000 Bq or 16.5 pg (picogram).[17]
ملاحظات
- ^ The density is calculated from the predicted metallic radius (Silva 2006) and the predicted close-packed crystal structure (Fournier 1976).
المراجع
- ^ أ ب ت ث Fournier, Jean-Marc (1976). "Bonding and the electronic structure of the actinide metals". Journal of Physics and Chemistry of Solids. 37 (2): 235–244. Bibcode:1976JPCS...37..235F. doi:10.1016/0022-3697(76)90167-0.
- ^ https://pubs.acs.org/doi/10.1021/jacs.8b09068
- ^ Haire, Richard G. (2006). "Einsteinium". In Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (PDF). Vol. 3 (3rd ed.). Dordrecht, the Netherlands: Springer. pp. 1577–1620. doi:10.1007/1-4020-3598-5_12. ISBN 978-1-4020-3555-5. Archived from the original (PDF) on 2010-07-17. Retrieved 2014-08-04.
- ^ أ ب Silva, pp. 1626–8
- ^ Johansson, Börje; Rosengren, Anders (1975). "Generalized phase diagram for the rare-earth elements: Calculations and correlations of bulk properties". Physical Review B. 11 (8): 2836–2857. Bibcode:1975PhRvB..11.2836J. doi:10.1103/PhysRevB.11.2836.
- ^ Hulet, E. K. (1980). "Chapter 12. Chemistry of the Heaviest Actinides: Fermium, Mendelevium, Nobelium, and Lawrencium". In Edelstein, Norman M. (ed.). Lanthanide and Actinide Chemistry and Spectroscopy. ACS Symposium Series. Vol. 131. pp. 239–263. doi:10.1021/bk-1980-0131.ch012. ISBN 9780841205680.
- ^ أ ب ت خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةSilva16345 - ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. pp. 4.121–4.123. ISBN 978-1439855119.
- ^ أ ب ت ث ج ح خ د ذ ر ز س ش ص Silva, pp. 1635–6
- ^ Toyoshima, Atsushi; Li, Zijie; Asai, Masato; Sato, Nozomi; Sato, Tetsuya K.; Kikuchi, Takahiro; Kaneya, Yusuke; Kitatsuji, Yoshihiro; Tsukada, Kazuaki; Nagame, Yuichiro; Schädel, Matthias; Ooe, Kazuhiro; Kasamatsu, Yoshitaka; Shinohara, Atsushi; Haba, Hiromitsu; Even, Julia (11 October 2013). "Measurement of the Md3+/Md2+ Reduction Potential Studied with Flow Electrolytic Chromatography". Inorganic Chemistry. 52 (21): 12311–3. doi:10.1021/ic401571h. PMID 24116851.
- ^ Silva, pp. 1633–4
- ^ Martin, W. C.; Hagan, Lucy; Reader, Joseph; Sugan, Jack (1974). "Ground Levels and Ionization Potentials for Lanthanide and Actinide Atoms and Ions" (PDF). J. Phys. Chem. Ref. Data. 3 (3): 771–9. Bibcode:1974JPCRD...3..771M. doi:10.1063/1.3253147. Archived from the original (PDF) on 2014-02-11. Retrieved 2013-10-19.
- ^ David R. Lide (ed), CRC Handbook of Chemistry and Physics, 84th Edition. CRC Press. Boca Raton, Florida, 2003; Section 10, Atomic, Molecular, and Optical Physics; Ionization Potentials of Atoms and Atomic Ions
- ^ أ ب ت خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةSilva16301 - ^ أ ب ت ث ج ح خ د ذ ر ز س ش Silva, pp. 1631–3
- ^ أ ب خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةbook2 - ^ Koch, Lothar (2000). "Transuranium Elements". Transuranium Elements, in Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a27_167. ISBN 978-3527306732.
ببليوگرافيا
- Silva, Robert J. (2006). "Fermium, Mendelevium, Nobelium, and Lawrencium" (PDF). In Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements. Vol. 3 (3rd ed.). Dordrecht: Springer. pp. 1621–1651. doi:10.1007/1-4020-3598-5_13. ISBN 978-1-4020-3555-5. Archived from the original (PDF) on 2010-07-17.
للاستزادة
- Hoffman, D.C., Ghiorso, A., Seaborg, G. T. The transuranium people: the inside story, (2000), 201–229
- Morss, L. R., Edelstein, N. M., Fuger, J., The chemistry of the actinide and transactinide element, 3, (2006), 1630–1636
- A Guide to the Elements – Revised Edition, Albert Stwertka, (Oxford University Press; 1998) ISBN 0-19-508083-1
المراجع
- معمل لوس ألاموس القومي - مندليفيوم
- دليل العناصر- طبعة منقحة, ألبرت ستيورت (طبعة جامعة أكسفورد,1998) ISBN 0-19-508083-1
- إنه أساسي- مندليفيوم
المصادر
- ويكيبيديا الإنجليزية .
