جسم مضاد وحيد النسيلة
الأجسام المضادة وحيدة النسيلة هي أجسام مُضادة أحادية النوعية هي نفسها تتكون من قبل خلايا المناعة التي هي كلها مُتطابقة من حيث الاستنساخ من الخلية الأم.
Monoclonal antibodies can have monovalent affinity, binding only to the same epitope (the part of an antigen that is recognized by the antibody). In contrast, polyclonal antibodies bind to multiple epitopes and are usually made by several different antibody-secreting plasma cell lineages. Bispecific monoclonal antibodies can also be engineered, by increasing the therapeutic targets of one monoclonal antibody to two epitopes.
It is possible to produce monoclonal antibodies that specifically bind to virtually any suitable substance; they can then serve to detect or purify it. This capability has become an important tool in biochemistry, molecular biology, and medicine. Monoclonal antibodies are being used on a clinical level for both the diagnosis and therapy of several diseases.[3] The administration of monoclonal antibodies was authorized by several countries for the treatment of moderate COVID-19 symptoms, however, it was concluded that there was almost no benefit in the use of mAb's in hospitalized COVID-19 patients.[4]
التاريخ
In the early 1900s, immunologist Paul Ehrlich proposed the idea of a Zauberkugel – "magic bullet", conceived of as a compound which selectively targeted a disease-causing organism, and could deliver a toxin for that organism. This underpinned the concept of monoclonal antibodies and monoclonal drug conjugates. Ehrlich and Élie Metchnikoff received the 1908 Nobel Prize for Physiology or Medicine for providing the theoretical basis for immunology.
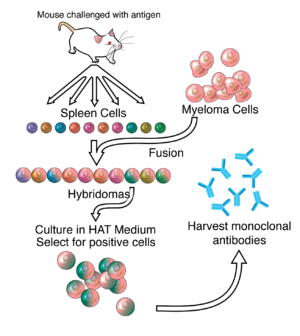
By the 1970s, lymphocytes producing a single antibody were known, in the form of multiple myeloma – a cancer affecting B-cells. These abnormal antibodies or paraproteins were used to study the structure of antibodies, but it was not yet possible to produce identical antibodies specific to a given antigen.[5] In 1973, Jerrold Schwaber described the production of monoclonal antibodies using human–mouse hybrid cells.[6] This work remains widely cited among those using human-derived hybridomas.[7] In 1975, Georges Köhler and César Milstein succeeded in making fusions of myeloma cell lines with B cells to create hybridomas that could produce antibodies, specific to known antigens and that were immortalized.[8] They and Niels Kaj Jerne shared the Nobel Prize in Physiology or Medicine in 1984 for the discovery.[8]
In 1988, Gregory Winter and his team pioneered the techniques to humanize monoclonal antibodies,[9] eliminating the reactions that many monoclonal antibodies caused in some patients. By the 1990s research was making progress in using monoclonal antibodies therapeutically, and in 2018, James P. Allison and Tasuku Honjo received the Nobel Prize in Physiology or Medicine for their discovery of cancer therapy by inhibition of negative immune regulation, using monoclonal antibodies that prevent inhibitory linkages.[10]
الانتاج


Hybridoma development
التنقية
Antibody heterogeneity
Recombinant
Chimeric antibodies
Human antibodies
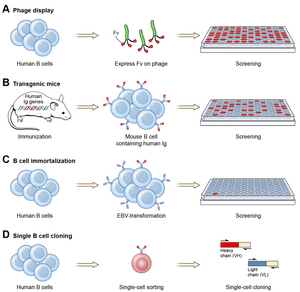
Ever since the discovery that monoclonal antibodies could be generated, scientists have targeted the creation of fully human products to reduce the side effects of humanised or chimeric antibodies. Several successful approaches have been identified: transgenic mice,[12] phage display[13] and single B cell cloning:[11]
As of November 2016, thirteen of the nineteen fully human monoclonal antibody therapeutics on the market were derived from transgenic mice technology.
التكلفة
Monoclonal antibodies are more expensive to manufacture than small molecules due to the complex processes involved and the general size of the molecules, all in addition to the enormous research and development costs involved in bringing a new chemical entity to patients. They are priced to enable manufacturers to recoup the typically large investment costs, and where there are no price controls, such as the United States, prices can be higher if they provide great value. Seven University of Pittsburgh researchers concluded, "The annual price of mAb therapies is about $100,000 higher in oncology and hematology than in other disease states," comparing them on a per patient basis, to those for cardiovascular or metabolic disorders, immunology, infectious diseases, allergy, and ophthalmology.[14]
تطبيقات
اختبارات تشخيصية
Once monoclonal antibodies for a given substance have been produced, they can be used to detect the presence of this substance. Proteins can be detected using the Western blot and immuno dot blot tests. In immunohistochemistry, monoclonal antibodies can be used to detect antigens in fixed tissue sections, and similarly, immunofluorescence can be used to detect a substance in either frozen tissue section or live cells.
استخدامات تحليلية وكيميائية
Antibodies can also be used to purify their target compounds from mixtures, using the method of immunoprecipitation.
استخدامات علاجية
Therapeutic monoclonal antibodies act through multiple mechanisms, such as blocking of targeted molecule functions, inducing apoptosis in cells which express the target, or by modulating signalling pathways.[15][16]
علاج السرطان
One possible treatment for cancer involves monoclonal antibodies that bind only to cancer-cell-specific antigens and induce an immune response against the target cancer cell. Such mAbs can be modified for delivery of a toxin, radioisotope, cytokine or other active conjugate or to design bispecific antibodies that can bind with their Fab regions both to target antigen and to a conjugate or effector cell. Every intact antibody can bind to cell receptors or other proteins with its Fc region.
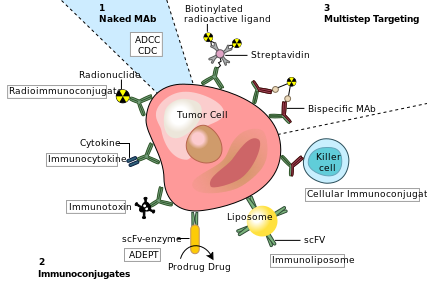
MAbs approved by the FDA for cancer include:[18]
أمراض المناعة الذاتية
Monoclonal antibodies used for autoimmune diseases include infliximab and adalimumab, which are effective in rheumatoid arthritis, Crohn's disease, ulcerative colitis and ankylosing spondylitis by their ability to bind to and inhibit TNF-α.[19] Basiliximab and daclizumab inhibit IL-2 on activated T cells and thereby help prevent acute rejection of kidney transplants.[19] Omalizumab inhibits human immunoglobulin E (IgE) and is useful in treating moderate-to-severe allergic asthma.
أمثلة للأجسام المضادة العلاجية وحيدة النسيلة ا
Below are examples of clinically important monoclonal antibodies.
| التصنيف الرئيسي | النوع | الاستخدام | الآلية/الهدف | Mode |
|---|---|---|---|---|
| Anti- inflammatory |
infliximab[19] | inhibits TNF-α | chimeric | |
| adalimumab | inhibits TNF-α | human | ||
| basiliximab[19] |
|
inhibits IL-2 on activated T cells | chimeric | |
| daclizumab[19] |
|
inhibits IL-2 on activated T cells | humanized | |
| omalizumab |
|
inhibits human immunoglobulin E (IgE) | humanized | |
| Anti-cancer | gemtuzumab[19] |
|
targets myeloid cell surface antigen CD33 on leukemia cells | humanized |
| alemtuzumab[19] | targets an antigen CD52 on T- and B-lymphocytes | humanized | ||
| rituximab[19] | targets phosphoprotein CD20 on B lymphocytes | chimeric | ||
| trastuzumab |
|
targets the HER2/neu (erbB2) receptor | humanized | |
| nimotuzumab |
|
EGFR inhibitor | Humanized | |
| cetuximab |
|
EGFR inhibitor | Chimeric | |
| bevacizumab |
|
inhibits VEGF | humanized | |
| Other | palivizumab[19] |
|
inhibits an RSV fusion (F) protein | humanized |
| abciximab[19] |
|
inhibits the receptor GpIIb/IIIa on platelets | chimeric |
اقرأ أيضًا
- قائمة الأجسام المضادة وحيدة النسيلة
- ترازتوموماب
- Antibody mimetic
- Immunotoxins, which sometimes use monoclonal antibodies as the targeting mechanism
- List of monoclonal antibodies
- Monoclonal antibody therapy
- Nomenclature of monoclonal antibodies
- Polyclonal antibodies
- Queen Mab Small mythological figure symbolizing hope (popular culture, used as biotech pun).
الهامش
- ^ "Cytochrome P450 Mediated Drug and Carcinogen Metabolism using Monoclonal Antibodies". home.ccr.cancer.gov. Retrieved 2 April 2018.
- ^ Gelboin HV, Krausz KW, Gonzalez FJ, Yang TJ (November 1999). "Inhibitory monoclonal antibodies to human cytochrome P450 enzymes: a new avenue for drug discovery". Trends in Pharmacological Sciences. 20 (11): 432–38. doi:10.1016/S0165-6147(99)01382-6. PMID 10542439.
- ^ Waldmann, Thomas A. (21 June 1991). "Monoclonal Antibodies in Diagnosis and Therapy". Science (in الإنجليزية). 252 (5013): 1657–1662. Bibcode:1991Sci...252.1657W. doi:10.1126/science.2047874. PMID 2047874. S2CID 19615695.
- ^ Shapiro, Adrienne E.; Ignacio, Rachel A. Bender (23 December 2021). "Time to knock monoclonal antibodies off the platform for patients hospitalised with COVID-19". The Lancet Infectious Diseases (in English). doi:10.1016/S1473-3099(21)00762-3. ISSN 1473-3099. PMC 8700277. PMID 34953521.
{{cite journal}}: CS1 maint: unrecognized language (link) - ^ Tansey EM, Catterall PP (July 1994). "Monoclonal antibodies: a witness seminar in contemporary medical history". Medical History. 38 (3): 322–27. doi:10.1017/s0025727300036632. PMC 1036884. PMID 7934322.
- ^ Schwaber J, Cohen EP (August 1973). "Human x mouse somatic cell hybrid clone secreting immunoglobulins of both parental types". Nature. 244 (5416): 444–47. doi:10.1038/244444a0. PMID 4200460. S2CID 4171375.
- ^ Cambrosio A, Keating P (1992). "Between fact and technique: the beginnings of hybridoma technology". Journal of the History of Biology. 25 (2): 175–230. doi:10.1007/BF00162840. PMID 11623041. S2CID 45615711.
- ^ أ ب Marks, LV. "The Story of César Milstein and Monoclonal Antibodies". WhatisBiotechnology.org. Retrieved 23 September 2020.
- ^ Riechmann L, Clark M, Waldmann H, Winter G (March 1988). "Reshaping human antibodies for therapy". Nature. 332 (6162): 323–27. Bibcode:1988Natur.332..323R. doi:10.1038/332323a0. PMID 3127726. S2CID 4335569.
- ^ Altmann DM (November 2018). "A Nobel Prize-worthy pursuit: cancer immunology and harnessing immunity to tumour neoantigens". Immunology. 155 (3): 283–84. doi:10.1111/imm.13008. PMC 6187215. PMID 30320408.
- ^ أ ب خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةHo_2018 - ^ Lonberg N, Huszar D (1995). "Human antibodies from transgenic mice". International Reviews of Immunology. 13 (1): 65–93. doi:10.3109/08830189509061738. PMID 7494109.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماة:1 - ^ Hernandez I, Bott SW, Patel AS, Wolf CG, Hospodar AR, Sampathkumar S, Shrank WH (February 2018). "Pricing of monoclonal antibody therapies: higher if used for cancer?". The American Journal of Managed Care. 24 (2): 109–12. PMID 29461857.
- ^ Breedveld FC (February 2000). "Therapeutic monoclonal antibodies". Lancet. 355 (9205): 735–40. doi:10.1016/S0140-6736(00)01034-5. PMID 10703815. S2CID 43781004.
- ^ Australian Prescriber (2006). "Monoclonal antibody therapy for non-malignant disease". Australian Prescriber. 29 (5): 130–33. doi:10.18773/austprescr.2006.079.
- ^ Modified from Carter P (November 2001). "Improving the efficacy of antibody-based cancer therapies". Nature Reviews. Cancer. 1 (2): 118–29. doi:10.1038/35101072. PMID 11905803. S2CID 10169378.
- ^ Takimoto CH, Calvo E. (1 January 2005) "Principles of Oncologic Pharmacotherapy" in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management
- ^ أ ب ت ث ج ح خ د ذ ر Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. pp. 241, for the examples infliximab, basiliximab, abciximab, daclizumab, palivusamab, gemtuzumab, alemtuzumab and rituximab, and mechanism and mode. ISBN 0-443-07145-4.
وصلات خارجية
- Monoclonal Antibodies Animation (Flash Required)
- Monoclonal Antibodies, from John W. Kimball's online biology textbook
- MeSH Monoclonal+antibodies
- Antibodypedia, open-access virtual repository publishing data and commentary on any antibodies available to the scientific community.