كربيد السيليكون

| |
| المُعرِّفات | |
|---|---|
| رقم CAS | |
| ECHA InfoCard | 100.006.357 |
PubChem CID
|
|
| رقم RTECS |
|
CompTox Dashboard (EPA)
|
|
| الخصائص | |
| الصيغة الجزيئية | SiC |
| كتلة مولية | 40.0962 g/mol |
| المظهر | شفاف إلى مسحوق أسود حسب النقاوة |
| الكثافة | 3.21 g/cm3 (جميع polytypes) [1] |
| نقطة الانصهار | |
| قابلية الذوبان في الماء | غير قابل للذوبان |
| قابلية الذوبان | غير قابل للذوبان في الأحماض |
| حركية الإلكترون | ~900 cm2/(V·s) (جميع الأنواع المتعددة) |
| معامل الانكسار (nD) | 2.55 (تحت الحمراء؛ جميع الأنواع المتعددة) [2] |
| المخاطر | |
تبويب الاتحاد الاوروپي (DSD)
|
غير مُدرَج |
| NFPA 704 (معيـَّن النار) | |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
كربيد السيليكون Silicon carbide (SiC)، ويعرف أيضاً بإسم كربورندم carborundum، هو مركب من السيليكون والكربون صيغته الكيميائية SiC. ويتواجد في الطبيعة في صيغة المعدن شديد الندرة مويسانيت moissanite. مسحوق كربيد السيليكون يـُنتـَج منذ 1893 للاستخدام كصنفرة. ويمكن لحبيبات كربيد السيليكون أن ترتبط معاً بالتحميص ليشكلوا أنواع من السيراميك شديدة الصلابة تـُستخدم على نطاق واسع في تطبيقات تتطلب تحمل عالي، مثل مكابح السيارات والألواح السيراميكية في السترات الواقية من الرصاص. التطبيقات الإلكترونية لكربيد السيليكون مثل المصباح الثنائي الباعث للضوء والكواشف في أجهزة الراديو المبكرة ظهرت حوالي 1907، والآن فإن كربيد السيليكون يـُستخدم على نطاق واسع في إلكترونيات أشباه الموصلات الحرارة العالية. البلورات المفردة الكبيرة من كربيد السيليكون يمكن الحصول عليهم بطريقة لـِلي؛ ويمكن قطعهم إلى جواهر تـُعرف بإسم "مويسانيت إصطناعي". ويمكن انتاج كربيد سيليكون بمساحة سطحية عالية من SiO2 المتواجد في مواد الأفران.
الاكتشاف والانتاج المبكر
ذُكرت توليفات مبكرة وغير منتظمة وغالباً ما تكون غير معروفة من كربيد السيليكون بواسطة دسپرتز (1849) ومارسدن (1880) وكولسون (1882).[3]
التواجد الطبيعي
يوجد المواسانيت بشكل طبيعي بكميات دقيقة فقط في أنواع معينة من النيازك وفي رواسب السامور والكمبرلايت. أما بالنسبة لكل كربيدات السيليكون المباعة في العالم، بما في ذلك مجوهرات المواسانيت، فهي اصطناعية بشكل تقريبي. كما عُثر على المواسانيت الطبيعي لأول مرة في عام 1893 كمكون صغير من نيزك كانيون ديابلو في أريزونا بواسطة دكتور فرديناند هنري مويسان، وبعد ذلك سُميت المادة في عام 1905.[4] كان اكتشاف مويسان للكربيد المتكون طبيعياً محل خلاف في البداية لأن عينته ربما تكون ملوثة بشفرات منشار كربيد السيليكون التي كانت موجودة بالفعل في السوق في ذلك الوقت.[5]
على الرغم من ندرة وجود كربيد السيليكون على الأرض، إلا أنه شائع بشكل ملحوظ في الفضاء. إنه شكل شائع من غبار النجوم الموجود حول النجوم الغنية بالكربون، وقد تم العثور على أمثلة لهذا الغبار النجمي في حالة بدائية في النيازك البدائية (غير المعدلة). يكاد يكون كربيد السيليكون الموجود في الفضاء والنيازك هو متعدد الأشكال بيتا. كما كشف تحليل حبيبات SiC الموجودة في نيزك مرتشيسون، نيزك كوندريت كربوني، عن نسب نظيرية شاذة للكربون والسيليكون، مما يشير إلى أن هذه الحبيبات نشأت خارج النظام الشمسي.[6]
الإنتاج
لأن المواسانيت الطبيعي نادر للغاية، فإن معظم كربيد السيليكون مصنوع من مواد صناعية. يستخدم كربيد السيليكون كمادة كاشطة، وكذلك لأشباه الموصلات وألماس المقلد لجودة الأحجار الكريمة. إن أبسط عملية لتصنيع كربيد السيليكون هي الجمع بين رمل السيليكا والكربون في فرن أتشيسون المقاوم للكهرباء من الگرافيت عند درجة حرارة عالية، بين 1،600 °C (2،910 °F) و2،500 °C (4،530 °F). يمكن تحويل جزيئات SiO الدقيقة SiO2 في المواد النباتية (مثل قشور الأرز) إلى SiC عن طريق تسخين الكربون الزائد من المادة العضوية.[7] غبار السيليكا، وهو منتج ثانوي لإنتاج معدن السيليكون وسبائك سليكون الحديد، يمكن أيضاً تحويله إلى كربيد SiC عن طريق التسخين باستخدام الگرافيت عند 1،500 °C (2،730 °F).[8]
تتنوع المادة المتكونة في فرن أتشسون من حيث النقاوة وفقاً لبعدها عن مصدر الحرارة المقاوم للگرافيت. تتميز البلورات عديمة اللون والأصفر والأخضر بأعلى درجة نقاء وهي الأقرب إلى المقاوم. ويتغير اللون إلى الأزرق والأسود عند مسافة أكبر من المقاوم، وتكون هذه البلورات الداكنة أقل نقاءً. يعتبر كل من النيتروجين والألمنيوم من الشوائب الشائعة، ويؤثران على التوصيل الكهربائي لـ SiC.[9]
يمكن صنع كربيد السيليكون النقي بواسطة عملية للي،[10] حيث يتم تسريع مسحوق SiC إلى أنواع عالية الحرارة من السيليكون والكربون وثاني كربيد السيليكون (SiC2) وكربيد السيليكون (Si2C) في محيط غاز الأرگون عند 2500 درجة مئوية وإعادة ترسيبها في بلورات مفردة تشبه الرقائق ،[11] يصل حجمها إلى 2 × 2 سم، في طبقة أساس أكثر برودة قليلاً. تنتج هذه العملية بلورات مفردة عالية الجودة، معظمها من طور 6H-SiC (بسبب ارتفاع درجة حرارة التطوير).
عملية للي المعدلة التي تتضمن تسخين بالتحريض في بوتقات الگرافيت تنتج بلورات مفردة أكبر يبلغ قطرها 4 بوصة (10 سم)، ولها قسم أكبر 81 مرة مقارنة بعملية للي التقليدية.[12]
عادةً ما تتم زراعة كربيد SiC المكعب بواسطة عملية أكثر تكلفة تتمثل في ترسيب كيميائي للبخار (CVD) من السيلان والهيدروجين والنيتروجين.[9][13] يمكن زراعة طبقات SiC متجانسة المحاور وغير متجانسة المحور باستخدام نهجي الطور الغازي والسائل.[14]
لتكوين كربيد السيليكون معقد الشكل، يمكن استخدام پوليمرات مسبق السيراميك كسلائف تشكل منتج السيراميك من خلال التحلل الحراري عند درجات حرارة في النطاق 1000–1100 °C.[15] تشتمل المواد الأولية للحصول على كربيد السيليكون بهذه الطريقة على بولي كربوسيلان وپولي(ميثيلسيلين) وپوليسيلازانات.[16] تُعرف مواد كربيد السيليكون التي تم الحصول عليها من خلال الانحلال الحراري پوليمرات مسبق السيراميك باسم السيراميك المشتق من الپوليمر أو PDCs. وغالباً ما يتم إجراء التحلل الحراري للبوليمرات السابقة للسيراميك بحكم جو خامل في درجات حرارة منخفضة نسبياً. بالنسبة لعملية CVD، فإن طريقة الانحلال الحراري مفيدة لأن الپوليمر يمكن أن يتشكل في أشكال مختلفة قبل التحويل الحراري إلى السيراميك.[17][18][19][20]
يمكن أيضاً تحويل SiC إلى رقائق عن طريق قطع بلورة واحدة إما باستخدام منشار سلك الماسي أو باستخدام الليزر. كما أن SiC هو أشباه موصلات مفيدة تستخدم في إلكترونيات الطاقة.[21]
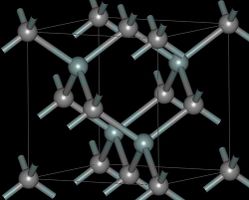
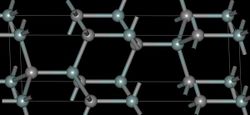
البنية والخصائص
- بنية الأشكال المتعددة لكربيد السيليكون.
| متعدد الأشكال | 3C (β) | 4H | 6H (α) |
|---|---|---|---|
| البنية البلورية | مزيج الزنك (مكعب) | مسدسة | مسدسة |
| زمرة فراغية | T2d-F43m | C46v-P63mc | C46v-P63mc |
| رمز پيرسون | cF8 | hP8 | hP12 |
| ثوابت العقد (Å) | 4.3596 | 3.0730; 10.053 | 3.0730; 15.11 |
| الكثافة (g/cm3) | 3.21 | 3.21 | 3.21 |
| فجوة النطاق (eV) | 2.36 | 3.23 | 3.05 |
| معامل الحجم (GPa) | 250 | 220 | 220 |
| الموصلية الحرارية (W/(cm·K)) | 3.6 | 3.7 | 4.9 |
كربيد السيليكون النقي عديم اللون. اللون البني إلى الأسود للمنتج الصناعي سببه شوائب الحديد. البريق المشابة لقوس قزح للبلورات تسببه طبقة التخميل من ثاني أكسيد السيليكون التي تتكون على السطح.
التوصيل الكهربائي
كربيد السيليكون هو شبه موصل، يمكن أن يكون مُطعَّم من النوع n بواسطة النيتروجين أو الفسفور والنوع p بواسطة البريليوم، البورون، الألمنيوم، أو الگاليوم. [2] كما تحققت الموصلية المعدنية عن طريق الجرعات الثقيلة بالبورون أو الألومنيوم أو النيتروجين.
اكتُشفت الموصلية الفائقة في 3C-SiC:Al، 3C-SiC:B و6H-SiC:B في درجات حرارة مماثلة ~ 1.5 كلڤن.[22][23]ومع ذلك، لوحظ اختلاف حاسم في سلوك المجال المغناطيسي بين الألومنيوم وتنشيط البورون: 3C-SiC: Al هو النوع الثاني. في المقابل ، 3C-SiC: B هي من النوع الأول، كما هو الحال مع 6H-SiC:B. وبالتالي يبدو أن خصائص الموصلية الفائقة تعتمد على المُطعم (البورون مقابل الألمنيوم) أكثر من اعتمادها على متعدد الأنواع (3C- مقابل 6H-). في محاولة لشرح هذا الاعتماد، لوحظ أن البدائل B في مواقع C في SiC، لكن Al في مواقع Si. لذلك، فإن Al و B "تدبر" بيئات مختلفة، في كلا النوعين.[24]
الاستخدامات
معدات الصنفرة والقطع
في الصناعة، يعتبر كربيد السيليكون مادة كاشطة شائعة في صناعة الجواهر بسبب المتانة والتكلفة المنخفضة للمادة. في التصنيع، يتم استخدامه لصلابته في المعالجة الآلية الكاشطة مثل التجليخ، الشحذ، القطع بالماء النفاث والسفع الرملي. يتم تصفيح جزيئات كربيد السيليكون على الورق لإنشاء ورق الصنفرة وشريط تثبيت على ألواح التزلج.[25]
في عام 1982 اكتُشف مركب قوي بشكل استثنائي من أكسيد الألمنيوم و شعيرات كربيد السيليكون. استغرق تطوير هذا المركب المنتج في المختبر إلى منتج تجاري ثلاث سنوات فقط. في عام 1985، تم تقديم أدوات القطع التجارية الأولى المصنوعة من مركب الألومينا وكربيد السيليكون المقوى بالشعيرات من قبل شركة المواد المركبة المتقدمة (ACMC) وشركة گرينليف.[26]
مادة هيكلية

في الثمانينيات والتسعينيات من القرن الماضي، تمت دراسة كربيد السيليكون في العديد من البرامج البحثية لتوربينات الغاز عالية الحرارة في أوروبا، اليابان والولايات المتحدة. تم تصميم المكونات لتحل محل شفرات التوربينات المصنوعة من سبائك النيكل الفائقة أو ريش الفوهة.[27] ومع ذلك، لم ينتج عن أي من هذه المشاريع كمية إنتاج، ويرجع ذلك أساساً إلى تأثير مقاومتها المنخفض ومتانة الكسر المنخفضة.[28]
مثل السيراميك الصلب الآخر (أي الألومينا وكربيد البورون)، يتم استخدام كربيد السيليكون في الدروع المركبة (على سبيل المثال درع تشوبهام)، وفي ألواح السيراميك في سترات واقية من الرصاص. أُنتج درع جلد التنين، بواسطة پيناكل للدروع، وعندها تم استخدام أقراص من كربيد السيليكون.[29] يمكن تسهيل مقاومة الكسر المحسنة في درع SiC من خلال ظاهرة نمو الحبيبات غير الطبيعي أو AGG. قد يعمل نمو حبيبات كربيد السيليكون الطويلة بشكل غير طبيعي على إضفاء تأثير مقوي من خلال سد الشقوق، على غرار تقوية الشعيرات. المشابه لتأثيرات تقوية AGG في نيتريد السيليكون (Si3N4).[30]
يستخدم كربيد السيليكون كمواد دعم ورفوف في أفران ذات درجة حرارة عالية مثل حرق السيراميك أو صهر الزجاج أو صب الزجاج. فرفوف فرن SiC أخف وزناً بكثير وأكثر متانة من رفوف الألومينا التقليدية.[31]
في ديسمبر 2015، تم ذكر حقن جزيئات كربيد السيليكون النانوية في المغنسيوم المنصهر كطريقة لإنتاج سبيكة بلاستيكية وقوية جديدة مناسبة للاستخدام في صناعة الطيران والفضاء والسيارات والإلكترونيات الدقيقة.[32]
قطع غيار السيارات
يتم استخدام مركب الكربون المدعم بألياف الكربون المُطعَّم من السليكون في "السيراميك" عالي الأداء المكابح القرصية، لأنها قادرة على تحمل درجات الحرارة القصوى. يتفاعل السيليكون مع الگرافيت في مركب الكربون المدعم بألياف الكربون ليصبح كربيد السيليكون المقوى بألياف الكربون (C/SiC). تُستخدم أقراص المكابح هذه في بعض السيارات الرياضية، والسيارات الخارقة، بالإضافة إلى سيارات الأداء الأخرى بما في ذلك بورش كاريرا جي تي، وبوگاتي ڤايرون، و شيڤروليه كورڤت ZR1، مكلارن پي1،[33]بنتلي، فيراري، لامبورگينى وبعض السيارات عالية الأداء كسيارات أودي. يستخدم كربيد السيليكون أيضاً في شكل متكلس من أجل مرشحات جسيمات الديزل.[34] كما أنها تستخدم كمضافات زيت[محل شك][مطلوب توضيح] لتقليل الاحتكاك والانبعاثات والتوافقيات.[35][36]
بوتقات المسابك
يستخدم SiC في البوتقات لحمل المعدن المنصهر في تطبيقات المسبك الصغيرة والكبيرة.[37][38]
الأنظمة الكهربائية
كان أول تطبيق كهربائي لـ SiC في مانع الصواعق في أنظمة الطاقة الكهربائية. يجب أن تُظهر هذه الأجهزة مقاومة عالية حتى يصل الجهد الكهربائي VT عبرها إلى حد معين وعند هذه النقطة يجب أن تنخفض مقاومتها إلى مستوى أقل وتحافظ على هذا المستوى حتى ينخفض الجهد المطبق إلى ما دون VT.[39]
أُدرك مبكراً في[when?] على أن SiC لها مقاومة تعتمد على الجهد، ولذا تم توصيل دعامات حبيبات SiC بين خطوط الطاقة عالية الجهد والأرض. عندما ترفع ضربة صاعقة للخط جهد الخط بشكل كافٍ، فإن عمود SiC سيوصل، مما يسمح لتيار الضربة بالمرور بشكل غير ضار إلى الأرض بدلاً من طول خط الطاقة. وقد أثبتت أعمدة SiC أنها تعمل بشكل كبير عند جهد التشغيل العادي لخط الطاقة، وبالتالي كان لا بد من وضعها في سلسلة مع فجوة شرارة. يتم تحجيم فجوة الشرارة هذه وجعلها موصلة عندما يرفع البرق جهد موصل خط الطاقة، وبالتالي يربط بشكل فعال دعامات SiC بين موصل الطاقة والأرض. لا يمكن الاعتماد على فجوات الشرارة المستخدمة في مانعات الصواعق، إما أن تفشل في ضرب قوس عند الحاجة أو تفشل في الإيقاف بعد ذلك، في الحالة الأخيرة بسبب فشل المواد أو تلوثها بالغبار أو الملح. كان الغرض من استخدام دعامات SiC في الأصل هو التخلص من الحاجة إلى فجوة الشرارة في مانعات الصواعق. تم استخدام موانع SiC الفجوة للحماية من الصواعق وتم بيعها بالأسماء التجارية GE و وستنگهاوس، من بين آخرين. تم إزاحة حاجز SiC المسدود إلى حد كبير عن طريق عدم وجود فجوة المقاومة المتغيرة مع الجهد تستخدم دعامات من حبيبات أكسيد الزنك.[40]
عناصر الدوائر الإلكترونية
كان كربيد السيليكون أول مادة شبه موصلة مهمة تجارياً. حصل الراديو البلوري على براءة اختراع كاشف "كربيد السيليكون" (كربيد السيليكون الصناعي) بواسطة هنري هاريسون تشيس دنوودي في عام 1906. وقد وجد استخدامه مبكراً في أجهزة الاستقبال على متن السفن.
الموصلية الكهربائية
كربيد السيليكون هو شبه موصل، والذي يمكن إعتباره من أنواع مركبات النيتروجين أوالفسفور و من أنواع الألومنيوم، والبورون، الغاليوم أو البريليوم [2] تُحقَّق الموصلية المعدنية عن طريق تطعيم المنشطات الثقيلة بالبورون أو الألومنيوم أو النيتروجين. تم الكشف عن الموصلية الفائقة فيC-SiC:Al، 3C-SiC:B و6H-SiC:B عند نفس درجة الحرارة البالغة 1.5 كلڤن.[23] أو الألمنيوم.[22]ومع ذلك، لوحظ اختلاف حاسم في سلوك المجال المغناطيسي بين الألومنيوم وتنشيط البورون: SiC:Al هو النوع الثاني، مثل Si:B. على العكس من ذلك، فإن SiC:B هي النوع الأول. في محاولة لشرح هذا الاختلاف، لوحظ أن مواقع Si أكثر أهمية من مواقع الكربون في الموصلية الفائقة في كربيد السيليكون. في حين أن البورون يحل محل الكربون في كربيد الصوديوم، فإن Al يحل محل مواقع Si. لذلك، فإن Al وB "يدبرون" بيئة مختلفة قد تفسر خصائص مختلفة لـ SiC: Al و SiC:B.[41]
أجهزة الطاقة الإلكترونية
كربيد السيليكون هو شبه موصل قيد البحث والإنتاج بالجملة في وقت مبكر والذي يوفر مزايا للأجهزة السريعة و/أو عالية الحرارة و/أو الجهد العالي. كانت الأجهزة الأولى المتاحة هي وصلة شوتكي، متبوعة بـ بوابة وصل ترانزستور تأثير المجال والموسفت للتبديل عالي الطاقة. تم تطوير الترانزستور ثنائي القطب والثايرستورات حالياً.[42]
كانت المشكلة الرئيسية لتسويق SiC هي التخلص من العيوب: خلع الحواف، والخلع اللولبي (الجوف والإصمات على حد سواء)، والعيوب المثلثية، وخلع المستوى القاعدي.[43] نتيجةً لذلك، أظهرت الأجهزة المصنوعة من بلورات SiC في البداية أداء منع عكسي ضعيف، على الرغم من أن الباحثين كانوا يجدون حلولًا مبدئية لتحسين أداء الانهيار.[44]بصرف النظر عن جودة البلورات، أعاقت مشاكل واجهة SiC مع ثاني أكسيد السيليكون تطوير الموسفتات القائمة على SiC والترانزستور ثنائي القطب ذو البوابة المعزولة. على الرغم من أن الآلية لا تزال غير واضحة، إلا أن نترجة المعادن قد قللت بشكل كبير من العيوب التي تسبب مشاكل الربط.[45]
في عام 2008، تم تقديم أول إعلان تجاري الترانزستور الحقلي الوصلي بمعدل 1200 V إلى السوق،[46] تبعها في عام 2011 أول موسفت تجاري تم تصنيفه عند 1200 V. JFETs متاحة الآن بمعدل 650 فولت إلى 1700 فولت مع مقاومة منخفضة تصل إلى 25 mΩ.[47] بجانب مفاتيح SiC وثنائيات SiC شوتكي (أيضاً الصمام الثنائي الحاجز شوتكي، SBD) في حزم TO-247 وTO-220 الشهيرة، بدأت الشركات حتى قبل ذلك في تنفيذ الرقائق المعزولة في وحدات الطاقة الإلكترونية الخاصة بها.
وجدت ثنائيات SiC SBD انتشاراً واسعاً في السوق يتم استخدامه في دوائر PFC ووحدة طاقة IGBT.[48] تقدم مؤتمرات مثل المؤتمر الدولي لأنظمة إلكترونيات الطاقة المتكاملة (CIPS) تقارير منتظمة حول التقدم التكنولوجي لأجهزة طاقة SiC. التحديات الرئيسية لإطلاق قدرات أجهزة طاقة SiC بالكامل هي:
- قدح البوابة: غالباً ما تتطلب أجهزة SiC مستويات جهد قادح بوابة تختلف عن نظيراتها من السيليكون وقد تكون غير متماثلة، على سبيل المثال،+20 V و−5 V.[49]
- التغليف: قد تحتوي رقائق SiC كثافة طاقة أعلى من أجهزة طاقة السيليكون وتكون قادرة على التعامل مع درجات حرارة أعلى تتجاوز حد السيليكون البالغ 150 درجة مئوية. وهناك حاجة لتقنيات إرفاق القالب الجديدة مثل التلبيد لإخراج الحرارة من الأجهزة بكفاءة وضمان اتصال موثوق به.[50]
الصمامات الثنائية الباعثة للضوء
اكتُشفت ظاهرة اللمعان الكهربائي في عام 1907 باستخدام كربيد السيليكون، وكان أول إعلان تجاه LEDs قائماً على SiC. تم تصنيع المصابيح الصفراء المصنوعة من 3C-SiC في الاتحاد السوڤيتي في السبعينيات[51] والمصباح الأزرق (6H-SiC) في جميع أنحاء العالم في الثمانينيات.[52]
سرعان ما توقف إنتاج LED عندما أظهرت مادة مختلفة، نيتريد الگاليوم، انبعاثاً أكثر سطوعاً بمقدار 10-100 مرة. يرجع هذا الاختلاف في الكفاءة إلى فجوة النطاق غير المباشرة من SiC، بينما يحتوي GaN على فجوة النطاق المباشرة التي تفضل انبعاث الضوء. ومع ذلك، لا يزال SiC أحد مكونات LED المهمة - فهو ركيزة شائعة لتنمية أجهزة GaN، كما أنه يعمل بمثابة موزع حراري في مصابيح LED عالية الطاقة.[52]
الفلك
معامل التمدد الحراري المنخفض والصلابة العالية والجساءة والتوصيل الحراري تجعل كربيد السيليكون مادة عاكسة مرغوبة للتلسكوبات الفلكية. تم تطوير تقنية النمو (الترسيب الكيميائي للبخار) لإنتاج أقراص من كربيد السيليكون متعدد الكريستالات يصل قطرها إلى 3.5 m (11 ft)، والعديد من التلسكوبات مثل مرصد هرشل الفضائي مجهز بالفعل ببصريات SiC،[53][54] بالإضافة إلى أنظمة المركبات الفضائية للمرصد الفضائي گايا مثبت على إطار صلب من كربيد السيليكون، والذي يوفر هيكلًا ثابتاً لن يتمدد أو ينكمش بسبب الحرارة.
علم اشتعال الفتائل الرفيعة
 مقالة مفصلة: علم اشتعال الفتائل الرفيعة
مقالة مفصلة: علم اشتعال الفتائل الرفيعة
تُستخدم ألياف كربيد السيليكون لقياس درجات حرارة الغاز في تقنية بصرية تسمى علم اشتعال الفتائل الرفيعة. يتضمن وضع فتيل رفيع في تيار غاز ساخن. يمكن أن ترتبط الانبعاثات الإشعاعية من الفتيل بدرجة حرارة الفتيل. الشعيرات هي ألياف كربونية يبلغ قطرها 15 ميكرومتراً، أي أرقّ بخمس مرات من شعر الإنسان. نظراً لأن الألياف رقيقة جداً، فإنها لا تفعل الكثير لبعثرة اللهب وتظل درجة حرارتها قريبة من درجة حرارة الغاز المحلي. ويمكن قياس درجات حرارة حوالي 800 - 2500 كلڤن.[55][56]
عناصر التسخين
توجد إشارات عناصر تسخين لكربيد السيليكون من أوائل القرن العشرين عندما تم إنتاجها بواسطة شركة أتشسنز كاربورندم في الولايات المتحدة وEKL في برلين. قدم كربيد السيليكون درجات حرارة تشغيل متزايدة مقارنةً بالسخانات المعدنية، على الرغم من أن درجة حرارة التشغيل كانت محدودة في البداية بواسطة أطراف مبردة بالماء، والتي جلبت التيار الكهربائي إلى المنطقة الساخنة من كربيد السيليكون. لم تكن المحطات متصلة بالمنطقة الساخنة، ولكنها كانت مثبتة في مكانها بواسطة الأوزان أو الربائع. تمت زيادة درجة حرارة التشغيل وكفاءته فيما بعد عن طريق استخدام كربيد السيليكون المنفصل ذو المقاومة المنخفضة "النهايات الباردة"، وعادة ما يكون قطرها أكبر من المنطقة الساخنة، ولكن لا يزال يتم تثبيته في مكانه عن طريق الضغط الميكانيكي فقط. وقد أدى تطوير تقنيات ربط التفاعل إلى إدخال العناصر المشتركة. في البداية، كانت تتميز بنهايات باردة ذات قطر أكبر، ولكن بحلول الأربعينيات من القرن الماضي، تم إنتاج عناصر ذات قطر متساوٍ. منذ الستينيات فصاعداً، تم إنتاج عناصر من قطعة واحدة، مع نهايات باردة تم إنشاؤها عن طريق ملء حجم المسام بسبيكة من السيليكون. أسلوب آخر من قطعة واحدة هو قطع فتحة لولبية في أنبوب متجانس حيث يكون القسم الساخن مطلوباً. كما تضمنت التطورات الأخرى إنتاج عناصر متعددة الأرجل، حيث يتم ربط ساقين أو أكثر بجسر مشترك، وإنتاج عناصر عالية الكثافة مرتبطة بالتفاعل، والتي توفر مقاومة إضافية اللأكسدة والهجوم الكيميائي. تُستخدم عناصر كربيد السيليكون اليوم في صهر المعادن غير الحديدية والزجاج، المعالجة الحرارية للمعادن، الألواح الزجاجية، وإنتاج السيراميك ومكونات الإلكترونيات، إلخ.[57]
عناصر الوقود النووي
غالباً ما يستخدم كربيد السيليكون كطبقة من الخواص ثلاثية التركيب لعناصر الوقود النووي لعناصر مفاعل تبريد بالغاز ذو درجة حرارة عالية أو مفاعل درجة حرارة عالية جداً مثل مفاعل مهد الحصى. كما يوفر كربيد السيليكون الاستقرار الميكانيكي للوقود وهو حاجز الانتشار الرئيسي لإطلاق نواتج الانشطار.[58]
المصوغات
باعتباره حجر كريم مستخدم في المجوهرات، يُطلق على كربيد السيليكون اسم "مواسانيت صناعي" أو "مويسانتي" بعد اسم المعدن. المويسانتي مشابه لألماس في عدة جوانب مهمة: إنه شفاف وصلب (9-9.5) على مقياس موز (مقارنة بـ 10 للماس)، مع معامل الانكسار بين 2.65 و2.69 (مقارنة بـ 2.42 للماس). مواسانيت أقسى إلى حد ما من الزركون المكعب الشائع. على عكس الماس، يمكن أن يكون المويسانتي بقوة مزدوجة الانكسار. هذه الجودة مرغوبة في بعض التطبيقات البصرية، ولكن ليس في الأحجار الكريمة. لهذا السبب، يتم قطع جواهر المويسانتي على طول المحور البصري من البلورة لتقليل تأثيرات الانكسار. إنه أخف وزناً (كثافة 3.21 g/cm3 مقابل 3.53 g/cm3)، وهو أكثر مقاومة للحرارة من الماس. ينتج عن هذا حجر لمعاناً أعلى وجوانب أكثر حدة ومرونة جيدة. يمكن وضع أحجار المويسانيت السائبة مباشرة في قوالب حلقة الشمع لصب الشمع المفقود؛ على عكس الماس، الذي يحترق عند 800 درجة مئوية، يظل المويسانيت غير متضرر بسبب درجات حرارة تصل إلى 1800 درجة مئوية (راجع درجة انصهار 1064 درجة مئوية الذهب). أصبح المويسانتي شائعاً كبديل للماس، وقد يُساء تعريفه على أنه الماس، نظراً لأن الموصلية الحرارية أقرب بكثير إلى الماس من أي بدائل أخرى للماس. يتم خداع العديد من أجهزة اختبار الماس الحراري بواسطة المويسانتي، ولكن يمكن تمييز الأحجار الكريمة عن الماس من خلال انكسارها مزدوج واللمعان الأخضر أو الأصفر الخفيف جداً تحت الضوء فوق البنفسجي. كما تحتوي بعض أحجار المويسانتي أيضاً على شوائب منحنية تشبه الأوتار، والتي لن يحتوي عليها الماس أبداً.[59]
انتاج الصلب
كربيد السيليكون المذاب في فرن الأكسجين القاعدي المستخدم في صنع الصلب يعمل بمثابة الوقود ويوفر الطاقة التي تزيد من نسبة الخردة إلى المعدن الساخن. يمكن استخدامه أيضاً لرفع درجات حرارة الصنبور وضبط محتوى الكربون. يكلف استخدام كربيد السيليكون أقل من تركيبة الفيروسيليكون والكربون، وينتج فولاذاً أنظف نظراً لانخفاض مستوى العناصر الشحيحة، وله محتوى غاز منخفض ولا يخفض من درجة حرارة الفولاذ.[60]
دعم المحفزات
أدت المقاومة الطبيعية للأكسدة التي أظهرها كربيد السيليكون، بالإضافة إلى اكتشاف طرق جديدة لتركيب شكل β-SiC المكعب، مع مساحة سطحه الأكبر، إلى اهتمام كبير باستخدامه كدعم محفز غير متجانس. تم بالفعل استخدام هذا النموذج كعامل محفز داعم لأكسدة الهيدروكربونات، مثل n-البيوتان، إلى أنهيدريد المالئيك.[61][62]
الطبع بالكربورندم
يستخدم كربيد السيليكون في الطبع بالكربورندم - تقنية الطباعة الفنية كولاگراف. يتم وضع حبيبات الكربورندم في عجينة على سطح لوح الألمنيوم. عندما يجف المعجون، يتم وضع الحبر واحتجازه في سطحه الحبيبي، ثم يمسح من المناطق العارية من اللوح. تُطبع لوحة الحبر بعد ذلك على الورق في مكبس ذو قاعدة دوارة يستخدم في الطباعة الغائرة. والنتيجة هي طباعة علامات مطلية منقوشة على الورق.[63]
يستخدم حصى الكربورندم أيضاً في الطباعة الحجرية. يسمح حجم الجسيمات المنتظم لها باستخدامها في "حبيبات" الحجر الذي يزيل الصورة السابقة. في عملية مماثلة للصنفرة، يتم وضع حبيبات الكربورندم الخشنة على الحجر وعمل باستخدام Levigator، ثم يتم تطبيق حصى أدق وأنعم تدريجياً حتى يصبح الحجر نظيفاً. هذا يخلق سطحاً حساساً للمواد الزيتية.[64]
إنتاج الگرافين
يمكن استخدام كربيد السيليكون في إنتاج الگرافين بسبب خواصه الكيميائية التي تعزز الإنتاج الفوقي للگرافين المحور على سطح الهياكل النانوية SiC.
عندما يتعلق الأمر بإنتاجه، يستخدم السيليكون أساساً كركيزة لتنمية الگرافين. ولكن هناك بالفعل عدة طرق يمكن استخدامها لتنمية الگرافين على كربيد السيليكون. تتكون طريقة نمو التكرر المتحكم فيه بالحصر (CCS) من شريحة SiC يتم تسخينها بفراغ باستخدام الگرافيت. ثم يتم تحرير الفراغ بشكل تدريجي للغاية للتحكم في نمو الگرافين. تنتج هذه الطريقة طبقات الگرافين الأعلى جودة. ولكن تم الإبلاغ عن طرق أخرى لإنتاج نفس المنتج أيضاً.
هناك طريقة أخرى لزيادة الگرافين وهي التحلل الحراري لـ SiC عند درجة حرارة عالية داخل الفراغ.[65]لكن تبين أن هذه الطريقة تنتج طبقات الگرافين التي تحتوي على حبيبات أصغر داخل الطبقات.[66]لذلك كانت هناك جهود لتحسين جودة وإنتاجية الگرافين. تتمثل إحدى هذه الطرق في إجراء رسم بياني خارج الموقع للسيليكون المنتهي SiC في جو يتكون من الأرجون. أثبتت هذه الطريقة أنها تنتج طبقات من الگرافين ذات نطاق أكبر من الطبقة التي يمكن تحقيقها عبر طرق أخرى. يمكن أن تكون هذه الطريقة الجديدة قابلة للتطبيق للغاية لإنتاج الگرافين عالي الجودة للعديد من التطبيقات التكنولوجية.
عندما يتعلق الأمر بفهم كيفية أو وقت استخدام هذه الأساليب لإنتاج الگرافين، فإن معظمهم ينتج أو ينمو هذا الگرافين بشكل أساسي على SiC ضمن بيئة تمكينية للنمو. يتم استخدامه غالباً في درجات حرارة أعلى (مثل 1300 درجة مئوية) بسبب الخصائص الحرارية SiC.[67] ومع ذلك، كانت هناك بعض الإجراءات التي تم إجراؤها ودراستها والتي من المحتمل أن تسفر عن طرق تستخدم درجات حرارة منخفضة للمساعدة في تصنيع الگرافين. وبشكل أكثر تحديداً، لوحظ أن هذا النهج المختلف لنمو الگرافين ينتج الجرافين في بيئة درجة حرارة تبلغ حوالي 750 درجة مئوية. تستلزم هذه الطريقة الجمع بين طرق معينة مثل ترسيب البخار الكيميائي (CVD) وعزل السطح. وعندما يتعلق الأمر بالركيزة، فإن الإجراء يتكون من طلاء ركيزة SiC بأغشية رقيقة من معدن انتقالي. وبعد المعالجة الحرارية السريعة لهذه المادة، ستصبح ذرات الكربون أكثر وفرة عند السطح البيني للفيلم المعدني الانتقالي الذي سينتج الگرافين بعد ذلك. ووجد أن هذه العملية تنتج طبقات گرافين أكثر استمرارية في جميع أنحاء سطح الركيزة.[68]
فيزياء الكم
يمكن أن يحتوي كربيد السيليكون على عيوب نقطية في الشبكة البلورية والتي تُعرف باسم مراكز الألوان. يمكن أن تنتج هذه العيوب فوتونات مفردة عند الطلب وبالتالي تعمل كمنصة لمصدر فوتون فردي. مثل هذا الجهاز هو مورد أساسي للعديد من التطبيقات الناشئة لعلوم المعلومات الكمومية. إذا قام أحدهم بضخ مركز ألوان عبر مصدر ضوئي خارجي أو تيار كهربائي، فسيتم إحضار مركز اللون إلى الحالة المثارة ثم التخفيف مع انبعاث فوتون فردي.[69][70]
عيب نقطة واحدة معروفة في كربيد السيليكون هو المطلق الذي له بنية إلكترونية مماثلة مثل مركز فراغ النيتروجين في الماس. في 4H-SiC، يحتوي الملئ على أربعة تكوينات مختلفة تتوافق مع أربعة خطوط خالية من الفونون (ZPL). تتم كتابة قيم ZPL هذه باستخدام الترميز VSi-VC والوحدة eV: hh(1.095), kk(1.096), kh(1.119)، وhk(1.150).[71]
انتاجه في العالم العربي
شراكة سعودية أمريكية يابانية
أعلنت في 9 مارس 2009 كل من شركة أحمد حمد القصيبي وإخوانه السعودية، وشركة واشنطن ميلز الأمريكية وشركة سوميتومو للمواد الصناعية اليابانية، عن توقيع مذكرة تفاهم لإنشاء مشروع مشترك لتصنيع مادة كربيد السيليكون في المملكة العربية السعودية، بتكلفة أولية تصل إلى 250 مليون ريال سعودي. وسيتم إنشاء المصنع في مدينة الجبيل الصناعية في المملكة العربية السعودية، وفقاً للدراسة الأولية للمشروع المتوقع استكماله في مطلع 2011، بطاقة إنتاجية أولية تصل إلى 24,000 طن من مادة كربيد السيليكون فائقة الجودة سنوياً. وبهذه المناسبة صرح السيد سعود عبد العزيز القصيبي، العضو المنتدب لشركة أحمد حمد القصيبي وإخوانه: "إن توقيع هذه المذكرة يعتبر إضافة قيّمة للاقتصاد والتنويع الصناعي السعودي، إذ أن المشروع سيعزز من فرص نشوء صناعات جديدة ويساهم في تعزيز الصادرات، بالإضافة إلى توفيرعدد كبير من فرص العمل الجديدة للشباب السعودي". وأضاف القصيبي قائلا:" شركاؤنا في مصنع كربيد السيليكون هم من رواد هذه الصناعةالمتميزين على المستوى العالمي، الأمر الذي سيؤدي إلى تبادل نافع للخبرات بين جميع الشركاء القائمين على المشروع لتطوير هذه الصناعة داخل المملكة".[72]
الهامش
- ^ P. Patnaik (2002). Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 0070494398.
- ^ أ ب ت ث "Properties of Silicon Carbide (SiC)". Ioffe Institute. Retrieved 2009-06-06.
- ^ A. W. Weimer (1997). Carbide, nitride, and boride materials synthesis and processing. Springer. p. 115. ISBN 0412540606.
- ^ Moissan, Henri (1904). "Nouvelles recherches sur la météorité de Cañon Diablo". Comptes rendus. 139: 773–86.
- ^ Di Pierro S.; Gnos E.; Grobety B.H.; Armbruster T.; Bernasconi S.M. & Ulmer P. (2003). "Rock-forming moissanite (natural α-silicon carbide)". American Mineralogist. 88 (11–12): 1817–21. Bibcode:2003AmMin..88.1817D. doi:10.2138/am-2003-11-1223. S2CID 128600868.
- ^ Kelly, Jim. "The Astrophysical Nature of Silicon Carbide". University College London. Archived from the original on May 4, 2017. Retrieved 2009-06-06.
- ^ Vlasov, A.S.; et al. (1991). "Obtaining silicon carbide from rice husks". Refractories and Industrial Ceramics. 32 (9–10): 521–523. doi:10.1007/bf01287542. S2CID 135784055.
- ^ Zhong, Y.; Shaw, Leon L.; Manjarres, Misael & Zawrah, Mahmoud F. (2010). "Synthesis of Silicon Carbide Nanopowder Using Silica Fume". Journal of the American Ceramic Society. 93 (10): 3159–3167. doi:10.1111/j.1551-2916.2010.03867.x.
- ^ أ ب Harris, Gary Lynn (1995). Properties of silicon carbide. IET. p. 19; 170–180. ISBN 978-0-85296-870-3.
- ^ Lely, Jan Anthony (1955). "Darstellung von Einkristallen von Silicium Carbid und Beherrschung von Art und Menge der eingebauten Verunreinigungen". Berichte der Deutschen Keramischen Gesellschaft. 32: 229–236.
- ^ Lely SiC Wafers. Nitride-crystals.com. Retrieved on 2013-05-04.
- ^ Ohtani, N.; et al. (2001). Nippon Steel Technical Report no. 84 : Large high-quality silicon carbide substrates (PDF). Archived from the original (PDF) on 2010-12-17.
- ^ Byrappa, K.; Ohachi, T. (2003). Crystal growth technology. Springer. pp. 180–200. ISBN 978-3-540-00367-0.
- ^ Bakin, Andrey S. (2006). "SiC Homoepitaxy and Heteroepitaxy". In M. Shur; S. Rumyantsev; M. Levinshtein (eds.). SiC materials and devices. Vol. 1. World Scientific. pp. 43–76. ISBN 978-981-256-835-9.
- ^ AM of Ceramics from Preceramic Polymers Published in Additive Manufacturing 2019, vol. 27 pp 80-90
- ^ Europe Makes Ceramics Preceramics
- ^ أ ب
Park, Yoon-Soo (1998). SiC materials and devices. Academic Press. pp. 20–60. ISBN 978-0-12-752160-2. خطأ استشهاد: وسم
<ref>غير صالح؛ الاسم "prop" معرف أكثر من مرة بمحتويات مختلفة. - ^ Pitcher, M. W.; Joray, S. J.; Bianconi, P. A. (2004). "Smooth Continuous Films of Stoichiometric Silicon Carbide from Poly(methylsilyne)". Advanced Materials. 16 (8): 706–709. doi:10.1002/adma.200306467.
- ^ Bunsell, A. R.; Piant, A. (2006). "A review of the development of three generations of small diameter silicon carbide fibres". Journal of Materials Science. 41 (3): 823–839. Bibcode:2006JMatS..41..823B. doi:10.1007/s10853-006-6566-z. S2CID 135586321.
- ^ Laine, Richard M.; Babonneau, Florence (1993). "Preceramic polymer routes to silicon carbide". Chemistry of Materials. 5 (3): 260–279. doi:10.1021/cm00027a007.
- ^ "KABRA|DISCO Corporation".
- ^ أ ب Muranaka, T.; Kikuchi, Yoshitake; Yoshizawa, Taku; Shirakawa, Naoki; Akimitsu, Jun (2008). "Superconductivity in carrier-doped silicon carbide". Sci. Technol. Adv. Mater. 9 (4): 044204. Bibcode:2008STAdM...9d4204M. doi:10.1088/1468-6996/9/4/044204. PMC 5099635. PMID 27878021. خطأ استشهاد: وسم
<ref>غير صالح؛ الاسم "muranaka" معرف أكثر من مرة بمحتويات مختلفة. - ^ أ ب Kriener, M.; Muranaka, Takahiro; Kato, Junya; Ren, Zhi-An; Akimitsu, Jun; Maeno, Yoshiteru (2008). "Superconductivity in heavily boron-doped silicon carbide". Sci. Technol. Adv. Mater. 9 (4): 044205. arXiv:0810.0056. Bibcode:2008STAdM...9d4205K. doi:10.1088/1468-6996/9/4/044205. PMC 5099636. PMID 27878022. خطأ استشهاد: وسم
<ref>غير صالح؛ الاسم "kriener" معرف أكثر من مرة بمحتويات مختلفة. - ^ Yanase, Y. & Yorozu, N. (2008). "Superconductivity in compensated and uncompensated semiconductors". Sci. Technol. Adv. Mater. 9 (4): 044201. Bibcode:2008STAdM...9d4201Y. doi:10.1088/1468-6996/9/4/044201. PMC 5099632. PMID 27878018.
- ^ Skateboard grip tape, United States Patent 5622759 (1997)
- ^ Narottam P. Bansal (2005). Handbook of ceramic composites. Springer. p. 312. ISBN 1402081332.
- ^ "Production of Silicon Carbide". siliconcarbide.net.
- ^ "Ceramics for turbine engines". unipass.com. Archived from the original on 2009-04-06. Retrieved 2009-06-06.
- ^ "Dragon Skin – Most Protective Body Armor – Lightweight". Future Firepower. Archived from the original on 2012-02-17. Retrieved 2009-06-06.
- ^ Abnormal Grain Growth in Journal of Crystal Growth 2012, Volume 359, Pages 83-91
- ^ "Silicon Carbide". Ceramic Arts Daily.
- ^ UCLA researchers create exceptionally strong and lightweight new metal
- ^ "Top 10 Fast Cars". topmost10.com. Archived from the original on 2009-03-26. Retrieved 2009-06-06.
- ^ O'Sullivan, D.; Pomeroy, M.J.; Hampshire, S.; Murtagh, M.J. (2004). "Degradation resistance of silicon carbide diesel particulate filters to diesel fuel ash deposits". MRS Proceedings. 19 (10): 2913–2921. Bibcode:2004JMatR..19.2913O. doi:10.1557/JMR.2004.0373.
- ^ "SiC Lubrication". Cerma.
- ^ Studt, P. (1987). "Influence of lubricating oil additives on friction of ceramics under conditions of boundary lubrication". Wear. 115 (1–2): 185–191. doi:10.1016/0043-1648(87)90208-0.
- ^ Friedrichs, Peter; Kimoto, Tsunenobu; Ley, Lothar; Pensl, Gerhard (2011). Silicon Carbide: Volume 1: Growth, Defects, and Novel Applications. John Wiley & Sons. pp. 49–. ISBN 978-3-527-62906-0.
- ^ Brown, John (1999). Foseco Non-Ferrous Foundryman's Handbook. Butterworth-Heinemann. pp. 52–. ISBN 978-0-08-053187-8.
- ^ Whitaker, Jerry C. (2005). The electronics handbook. CRC Press. p. 1108. ISBN 978-0-8493-1889-4.
- ^ Bayliss, Colin R. (1999). Transmission and distribution electrical engineering. Newnes. p. 250. ISBN 978-0-7506-4059-6.
- ^ Yanase, Y. and Yorozu, N. (2008). "Superconductivity in compensated and uncompensated semiconductors" (free download). Sci. Technol. Adv. Mater. 9 (4): 044201. doi:10.1088/1468-6996/9/4/044201.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bhatnagar, M.; Baliga, B.J. (March 1993). "Comparison of 6H-SiC, 3C-SiC, and Si for power devices". IEEE Transactions on Electron Devices. 40 (3): 645–655. Bibcode:1993ITED...40..645B. doi:10.1109/16.199372.
- ^ Chen, H.; Raghothamachar, Balaji; Vetter, William; Dudley, Michael; Wang, Y.; Skromme, B.J. (2006). "Effects of defect types on the performance of devices fabricated on a 4H-SiC homoepitaxial layer". Mater. Res. Soc. Symp. Proc. 911: 169. doi:10.1557/PROC-0911-B12-03.
- ^ Madar, Roland (26 أغسطس 2004). "Materials science: Silicon carbide in contention". Nature. 430 (7003): 974–975. Bibcode:2004Natur.430..974M. doi:10.1038/430974a. PMID 15329702. S2CID 4328365.
- ^ Chen, Z.; Ahyi, A.C.; Zhu, X.; Li, M.; Isaacs-Smith, T.; Williams, J.R.; Feldman, L.C. (2010). "MOS Characteristics of C-Face 4H-SiC". J. Of Elec. Mat. 39 (5): 526–529. Bibcode:2010JEMat..39..526C. doi:10.1007/s11664-010-1096-5. S2CID 95074081.
- ^ "At 1200 V and 45 milliohms, SemiSouth introduces the industry's lowest resistance SiC power transistor for efficient power management". Reuters (Press release). 5 مايو 2011. Archived from the original on 15 مارس 2016.
- ^ "SiC JFETs Archives". United Silicon Carbide Inc. (in الإنجليزية). Retrieved 2021-01-11.
- ^ "Cree launches industry's first commercial silicon carbide power MOSFET; destined to replace silicon devices in high-voltage (≥ 1200 V) power electronics" (Press release). Cree. 17 يناير 2011.
- ^ Meißer, Michael (2013). Resonant Behaviour of Pulse Generators for the Efficient Drive of Optical Radiation Sources Based on Dielectric Barrier Discharges. KIT Scientific Publishing. p. 94. ISBN 978-3-7315-0083-4.
- ^ Horio, Masafumi; Iizuka, Yuji; Ikeda, Yoshinari (2012). "Packaging Technologies for SiC Power Modules" (PDF). Fuji Electric Review. 58 (2): 75–78.
- ^ Klipstein, Don. "Yellow SiC LED". Retrieved 6 يونيو 2009.
- ^ أ ب Stringfellow, Gerald B. (1997). High brightness light emitting diodes. Academic Press. pp. 48, 57, 425. ISBN 978-0-12-752156-5.
- ^ "The largest telescope mirror ever put into space". European Space Agency. Retrieved 2009-06-06.
- ^ Petrovsky, Gury T.; Tolstoy, Michael N.; Lubarsky, Sergey V.; Khimitch, Yuri P.; Robb, Paul N.; Tolstoy; Lubarsky; Khimitch; Robb (1994). Stepp, Larry M. (ed.). "2.7-meter-diameter silicon carbide primary mirror for the SOFIA telescope". Proc. SPIE. Advanced Technology Optical Telescopes V. 2199: 263. Bibcode:1994SPIE.2199..263P. doi:10.1117/12.176195. S2CID 120854083.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Thin-Filament Pyrometry Developed for Measuring Temperatures in Flames". NASA. Retrieved 2009-06-06.
- ^ Maun, Jignesh D. (2007). "Thin-filament pyrometry with a digital still camera". Applied Optics. 46: 483. doi:10.1364/AO.46.000483.
- ^ Yeshvant V. Deshmukh (2005). Industrial heating: principles, techniques, materials, applications, and design. CRC Press. p. 383-393. ISBN 0849334055.
- ^ E. López-Honorato; et al. (2009). "TRISO coated fuel particles with enhanced SiC properties". Journal of Nuclear Materials. 392: 219. doi:10.1016/j.jnucmat.2009.03.013.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ M. O'Donoghue (2006). Gems. Elsevier. p. 89. ISBN 0-75-065856-8.
- ^ "Silicon carbide (steel industry)". Retrieved 2009-06-06.
- ^ Howard F. Rase (2000). Handbook of commercial catalysts: heterogeneous catalysts. CRC Press. p. 258. ISBN 0849394171.
- ^ "High surface area silicon carbide from rice husk: A support material for catalysts".
{{cite journal}}: Cite journal requires|journal=(help) - ^ "Printmaking". Retrieved 2009-07-31.
- ^ "Printmaking". Bircham Gallery, birchamgallery.co.uk. Retrieved 2009-07-31.
- ^ Ruan, Ming; Hu, Yike; Guo, Zelei; Dong, Rui; Palmer, James; Hankinson, John; Berger, Claire; Heer, Walt A. de (December 2012). "Epitaxial graphene on silicon carbide: Introduction to structured graphene" (PDF). MRS Bulletin (in الإنجليزية). 37 (12): 1138–1147. doi:10.1557/mrs.2012.231. ISSN 0883-7694.
- ^ Emtsev, Konstantin V.; Bostwick, Aaron; Horn, Karsten; Jobst, Johannes; Kellogg, Gary L.; Ley, Lothar; McChesney, Jessica L.; Ohta, Taisuke; Reshanov, Sergey A. (2009-02-08). "Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide". Nature Materials (in الإنجليزية). 8 (3): 203–207. Bibcode:2009NatMa...8..203E. doi:10.1038/nmat2382. hdl:11858/00-001M-0000-0010-FA05-E. ISSN 1476-1122. PMID 19202545.
- ^ de Heer, Walt A.; Berger, Claire; Wu, Xiaosong; First, Phillip N.; Conrad, Edward H.; Li, Xuebin; Li, Tianbo; Sprinkle, Michael; Hass, Joanna (July 2007). "Epitaxial graphene". Solid State Communications. 143 (1–2): 92–100. arXiv:0704.0285. Bibcode:2007SSCom.143...92D. doi:10.1016/j.ssc.2007.04.023. ISSN 0038-1098. S2CID 44542277.
- ^ Juang, Zhen-Yu; Wu, Chih-Yu; Lo, Chien-Wei; Chen, Wei-Yu; Huang, Chih-Fang; Hwang, Jenn-Chang; Chen, Fu-Rong; Leou, Keh-Chyang; Tsai, Chuen-Horng (2009-07-01). "Synthesis of graphene on silicon carbide substrates at low temperature". Carbon (in الإنجليزية). 47 (8): 2026–2031. doi:10.1016/j.carbon.2009.03.051. ISSN 0008-6223.
- ^ Lohrmann, A.; Iwamoto, N.; Bodrog, Z.; Castalletto, S.; Ohshima, T.; Karle, T.J.; Gali, A.; Prawer, S.; McCallum, J.C.; Johnson, B.C. (2015). "Single-photon emitting diode in silicon carbide". Nature Communications. 6: 7783. arXiv:1503.07566. Bibcode:2015NatCo...6.7783L. doi:10.1038/ncomms8783. PMID 26205309. S2CID 205338373.
- ^ Khramtsov, I.A.; Vyshnevyy, A.A.; Fedyanin, D. Yu. (2018). "Enhancing the brightness of electrically driven single-photon sources using color centers in silicon carbide". NPJ Quantum Information. 4: 15. Bibcode:2018npjQI...4...15K. doi:10.1038/s41534-018-0066-2.
- ^ Davidsson, J.; Ivády, V.; Armiento, R.; Son, N.T.; Gali, A.; Abrikosov, I. A. (2018). "First principles predictions of magneto-optical data for semiconductor point defect identification: the case of divacancy defects in 4H–SiC". New Journal of Physics. 20 (2): 023035. arXiv:1708.04508. Bibcode:2018NJPh...20b3035D. doi:10.1088/1367-2630/aaa752. S2CID 4867492.
- ^ "شراكة سعودية أمريكية يابانية لتصنيع مادة كربيد السيليكون". جريدة الرياض. 2009-03-10. Retrieved 2009-12-18.
وصلات خارجية
- Silicon Carbide web book
- A Brief History of Silicon Carbide Dr J F Kelly, University of London
- Material Safety Data Sheet for Silicon Carbide
- Moissanite on Mindat.org
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Short description is different from Wikidata
- Articles with hatnote templates targeting a nonexistent page
- مقالات ذات عبارات محل شك
- جميع الصفحات التي تحتاج تنظيف
- مقالات بالمعرفة تحتاج توضيح from December 2021
- Vague or ambiguous time from December 2021
- Diamond simulants
- أحجار كريمة
- أشباه موصلات المجموعة الرابعة
- كربيدات
- مركبات السيليكون
- مركبات سيليكون غير عضوية
- مواد شبه موصلة
- مواد فائقة الصلابة
- مواد سيراميكية
- مواد حرارية
- معادن اصطناعية
- مزيلات الأكسدة
- كواشط