هافنيوم
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| صفات عامة | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| الإسم, الرقم, الرمز | هافنيوم, Hf, 72 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| سلاسل كيميائية | فلزات انتقالية | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| المجموعة, الدورة, المستوى الفرعي | d , 6 , 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| المظهر | فولاذي رمادي 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| كتلة ذرية | 178.49(2) g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| شكل إلكتروني | [Xe] 6s2 4f14 5d2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| عدد الإلكترونات لكل مستوى | 2, 8, 18, 32, 10, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| خواص فيزيائية | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الحالة | صلب | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| كثافة عندح.غ. | 13.31 ج/سم³ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| كثافة السائل عند m.p. | 12 ج/سم³ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| نقطة الإنصهار | 2506 ك 2233 م ° 4051 ف ° | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| نقطة الغليان | 4876 ك 4603 م ° 8317 ف ° | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| حرارة الإنصهار | kJ/mol 27.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| حرارة التبخر | kJ/mol 571 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| السعة الحرارية | (25 25.73 C (م) ° ( J/(mol·K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الخواص الذرية | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| البنية البللورية | سداسية | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| حالة التأكسد | 4 (اكسيد أمفوتيري) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| سالبية كهربية | 1.3 (مقياس باولنج) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| طاقة التأين (المزيد) |
1st: 658.5 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1440 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 2250 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| نصف قطر ذري | 155 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| نصف قطر ذري (حسابيا) | 208 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| نصف القطر التساهمي | 150 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| متفرقة | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الترتيب المغناطيسي | no data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| مقاومة كهربية | 20 °C 331 nΩ·m | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| توصيل حراري | (300 K ك ) 23.0 (W/(m·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| تمدد حراري | (25 °C) 5.9 µm/(m·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| سرعة الصوت (قضيب رفيع) | (20 °م) 3010 m/s | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| معامل يونج | 78 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| معامل القص | 30 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| معاير الحجم | 110 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| نسبة بواسون | 0.37 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| صلابة موس | 5.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| رقم فيكرز للصلادة | 1760 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| رقم برينل للصلادة | 1700 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| رقم التسجيل | 7440-58-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| النظائر المهمة | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| المراجع | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
هافنيوم Hafnium (ونطقه حسب IPA: /ˈhæfniəm/) هو عنصر كيميائي في الجدول الدوري ورمز Hf وله رقم ذري 72. والهافنيوم فلز انتقالي براق، رمادي فضي رباعي التكافؤ. الهافنيوم يشابه كيميائياً الزركنيوم ويتواجد في أملاح الزركونيوم. ويستخدم الهافنيوم في سبائك التنجستن في الفتائل والإلكترودات ويعمل كممتص للنيوترونات في قضبان التحكم في مفاعلات الطاقة النووية.
وهو معدن له مظهر الفولاذ، رمزه الكيمياوي Hf، عدده الذري 72؛ وهو أحد عناصر الفصيلة الثانوية IVB (أو 4) التي تضم إضافة إليه التيتانيوم والزركونيوم والرذرفورديوم. تحوي ذرته في طبقتها الإلكترونية الخارجية الكترونين s وفي الطبقة التي تليها إلكترونين d. وعليه فإن تكافؤه يجب أن يأخذ - نظرياً- القيم من 1 إلى 4؛ إلا أن هذا لا يتحقق عملياً إذ لا تعرف للهفنيوم في تكافؤاته 1 و2 و3 سوى مركبات قليلة العدد. فثبات التكافؤات الدنيا يتناقص من التيتانيوم حتى الهفنيوم مع ازدياد ثبات التكافؤ (4). كما تتناقص شدة الخاصة الحمضية لأكاسيدها بالترتيب نفسه مع ازدياد الخاصة الأساسية.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
الخصائص
الخصائص الطبيعية
Hafnium is a shiny, silvery, ductile metal that is corrosion-resistant and chemically similar to zirconium[1] (due to its having the same number of valence electrons, being in the same group, but also to relativistic effects; the expected expansion of atomic radii from period 5 to 6 is almost exactly cancelled out by the lanthanide contraction). Hafnium changes from its alpha form, a hexagonal close-packed lattice, to its beta form, a body-centered cubic lattice, at 2388 K.[2] The physical properties of hafnium metal samples are markedly affected by zirconium impurities, especially the nuclear properties, as these two elements are among the most difficult to separate because of their chemical similarity.[1]
A notable physical difference between these metals is their density, with zirconium having about one-half the density of hafnium. The most notable nuclear properties of hafnium are its high thermal neutron capture cross section and that the nuclei of several different hafnium isotopes readily absorb two or more neutrons apiece.[1] In contrast with this, zirconium is practically transparent to thermal neutrons, and it is commonly used for the metal components of nuclear reactors – especially the cladding of their nuclear fuel rods.
الخصائص الكيميائية
Hafnium reacts in air to form a protective film that inhibits further corrosion. The metal is not readily attacked by acids but can be oxidized with halogens or it can be burnt in air. Like its sister metal zirconium, finely divided hafnium can ignite spontaneously in air. The metal is resistant to concentrated alkalis.
As a consequence of lanthanide contraction, the chemistry of hafnium and zirconium is so similar that the two cannot be separated on the basis of differing chemical reactions. The melting points and boiling points of the compounds and the solubility in solvents are the major differences in the chemistry of these twin elements.[3]
درجة أكسدته الأثبت +4، وتتأكسد مركّباته بدرجات الأكسدة الأخرى (+2 و+3) بسرعة متحولة إلى MIV بفعل الهواء أو الماء أو مواد أخرى. وتكون الطاقة اللازمة لإزاحة أربعة إلكترونات كبيرة؛ لذا لا وجود للأيون M+4، فمركّباته بدرجة الأكسدة +4 ذات طبيعة مشتركة.
لذرتي الهافنيوم والزركونيوم نصفا قطر متماثلان، كما أن لأيونيها نصفي قطر متماثلين لانكماش نصف القطر الذري الحاصل في زمرة اللانتانيوم. لذلك كان السلوك الكيمياوي لهما متشابهاً لدرجة كبيرة، فهما يتشابهان أكثر من أي عنصرين آخرين في الجدول الدوري.
الهافنيوم خامل كيمياوياً عند الدرجة العادية من الحرارة. أما عند الدرجات المرتفعة فهو شديد النشاط؛ إذ لا يقتصر نشاطه على التفاعل مع الهالوجينات مكوناً الهاليدات MX4 والأكسجين والكبريت فقط، وإنما يتفاعل مع الكربون والآزوت (النتروجين) كذلك. وهو يحترق في جو من الأكسجين عند تسخينه إلى درجة التوهج (أعلى من 600 ْس).
النظائر
At least 36 isotopes of hafnium have been observed, ranging in mass number from 153 to 188.[4][5] The five stable isotopes are in the range of 176 to 180. The radioactive isotopes' half-lives range from 400 ms for 153Hf[5] to 7.0×1016 years for the most stable one, the primordial 174Hf.[4][6]
The extinct radionuclide 182Hf has a half-life of 8.9±0.1 million years, and is an important tracker isotope for the formation of planetary cores.[7] The nuclear isomer 178m2Hf was at the center of a controversy for several years regarding its potential use as a weapon.
التواجد
Hafnium is estimated to make up about 5.8 ppm of the Earth's upper crust by mass. It does not exist as a free element on Earth, but is found combined in solid solution with zirconium in natural zirconium compounds such as zircon, ZrSiO4, which usually has about 1–4% of the Zr replaced by Hf. Rarely, the Hf/Zr ratio increases during crystallization to give the isostructural mineral hafnon (Hf,Zr)SiO
4, with atomic Hf > Zr.[8] An obsolete name for a variety of zircon containing unusually high Hf content is alvite.[9]
A major source of zircon (and hence hafnium) ores is heavy mineral sands ore deposits, pegmatites, particularly in Brazil and Malawi, and carbonatite intrusions, particularly the Crown Polymetallic Deposit at Mount Weld, Western Australia. A potential source of hafnium is trachyte tuffs containing rare zircon-hafnium silicates eudialyte or armstrongite, at Dubbo in New South Wales, Australia.[10]
يصادف الهافنيوم غالباً في فلزات الزركونيوم بنسبة 1-3٪، والزركونيوم تبلغ نسبته في القشرة الأرضية 0.025٪ وزناً، ويأتي في المرتبة الخامسة والأربعين بسعة انتشاره بين العناصر في القشرة الأرضية.
الزركونيوم والهافنيوم متشابهان كيمياوياً شبهاً كبيراً، ولهذا فهما يحضّران معاً، ومن ثم يفصل أحدهما عن الآخر.
بنيته الإلكترونية [Xe]6s2 4f14 5d2 أو 2-8-18-32-10-2، كتلته الذرية النسبية: 178.49، كتلته الحجمية 13.31غ/سم3، درجة انصهاره 2222 ْس، درجة غليانه 5200 ْس، نصف قطر ذرته 158.05 بيكومتر، نصف قطر الأيون Hf4+ ن84 بيكومتر، وهو يقبل الطرق والسحب.
يستعمل الهافنيوم بسبب ارتفاع درجة انصهاره وشدة إشعاعه للإلكترونات في صنع مساري المصابيح الإلكترونية وأشرطة التوهج، كما أنه يدخل في تركيب سبائك ذات درجات انصهار عالية مع الحديد والنيكل، وهو يستعمل في التقانة الإلكترونية بسبب شدة تفاعله مع النترونات، ويستعمل أكسيده HfO2 لصنع زجاج ذي قرينة انكسار عالية. سبيكته Ta4HfC5 لها أعلى درجة انصهار بين المواد المعروفة جميعها (4215 ْس) وسبيكته (88٪ تنتاليوم و9.6٪ تنجستن و2.4٪ هفنيوم) غالية جداً؛ إذ إنها تستعمل في مجال واسع من درجات الحرارة.
الانتاج

The heavy mineral sands ore deposits of the titanium ores ilmenite and rutile yield most of the mined zirconium, and therefore also most of the hafnium.[11]
Zirconium is a good nuclear fuel-rod cladding metal, with the desirable properties of a very low neutron capture cross section and good chemical stability at high temperatures. However, because of hafnium's neutron-absorbing properties, hafnium impurities in zirconium would cause it to be far less useful for nuclear-reactor applications. Thus, a nearly complete separation of zirconium and hafnium is necessary for their use in nuclear power. The production of hafnium-free zirconium is the main source for hafnium.[1]

The chemical properties of hafnium and zirconium are nearly identical, which makes the two difficult to separate.[12] The methods first used — fractional crystallization of ammonium fluoride salts[13] or the fractional distillation of the chloride[14] — have not proven suitable for an industrial-scale production. After zirconium was chosen as material for nuclear reactor programs in the 1940s, a separation method had to be developed. Liquid–liquid extraction processes with a wide variety of solvents were developed and are still used for the production of hafnium.[15] About half of all hafnium metal manufactured is produced as a by-product of zirconium refinement. The end product of the separation is hafnium(IV) chloride.[16] The purified hafnium(IV) chloride is converted to the metal by reduction with magnesium or sodium, as in the Kroll process.[17]
Further purification is effected by a chemical transport reaction developed by Arkel and de Boer: In a closed vessel, hafnium reacts with iodine at temperatures of 500 °C (900 °F), forming hafnium(IV) iodide; at a tungsten filament of 1,700 °C (3,100 °F) the reverse reaction happens preferentially, and the chemically bound iodine and hafnium dissociate into the native elements. The hafnium forms a solid coating at the tungsten filament, and the iodine can react with additional hafnium, resulting in a steady iodine turnover and ensuring the chemical equilibrium remains in favor of hafnium production.[3][18]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
المركبات الكيميائية
الأكسيد HfO2 مادة بيضاء صعبة الانصهار والانحلال في الماء أو في الحموض والأسس الممددة. ينحل بسهولة في HF أو القلويات المصهورة. ولصعوبة انحلال الأكسيد MO2 في الماء لايمكن الحصول على الهدروكسيد Hf(OH)4 مباشرة، فيحصل عليه مثلاً بتفاعل القلويات مع الهاليدات مثل HfCl4، وتغلب على أكسيده الخواص الأساسية، فالهدروكسيد Hf(OH)4 ينحل في الحموض القوية.[19]
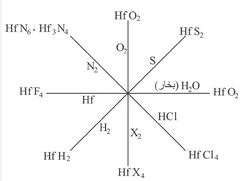
هاليداته لها الصيغة العامة HfX4 ويحصل عليها بحرق مزيج من الأكسيد الموافق للمعدن مع الكربون في جو من الهالوجين. ويحدث التفاعل حسب المعادلة:
رباعي كلوريد الهفنيوم ملح نموذجي، وجميع هاليدات الهفنيوم تنحل جيداً بالماء باستثناء HfF4. وتكوِّن هاليداته جميعها معقدات[ر: المعقَّد] صيغتها العامة A2[HfX6] حيث A معدن أحادي التكافؤ. وهذه المعقدات تتبلور جيداً، وتتحلمه (تتحلل بالماء) أقل بكثير من الهاليدات البسيطة؛ مما يدل على ثبات الأيونات المعقّدة [MX6]2- في المحاليل. معقداته جميعها لا لون لها. وتتناقص درجة ثبات هاليداته ابتداء من الفلور حتى اليود. ويكوِّن الهفنيوم - إضافة إلى المعقَّدات A2[HfX6] - عقدات أخرى صيغتها: A3[HfF7] وA4[HfF8]، وA5[HfF9] حيث يكون العدد التساندي فيها 7 و8 و9 على الترتيب.
التاريخ
اكتشفه عام 1923 الكيميائي المجري گيورگ ڤون هڤسي Georg von Hevesy ت(1885-1966) بالاشتراك مع الفيزيائي الهولندي ديرك كوستر Dirk Coster، وكان ذلك بعد قرن تقريباً من اكتشاف الزركونيوم عام 1789. وذلك بعد أن تنبأ نيلز بور Niels Bohr أن العنصر 72 سيشبه الزركونيوم في بنيته، ولذلك بحث مكتشفاه عنه في خامات الزركونيوم. واشتق اسمه من هافنيا Hafnia الاسم اللاتيني كوپنهاگن حيث اكتشف العنصر.
In his report on The Periodic Law of the Chemical Elements, in 1869, Dmitri Mendeleev had implicitly predicted the existence of a heavier analog of titanium and zirconium. At the time of his formulation in 1871, Mendeleev believed that the elements were ordered by their atomic masses and placed lanthanum (element 57) in the spot below zirconium. The exact placement of the elements and the location of missing elements was done by determining the specific weight of the elements and comparing the chemical and physical properties.[20]
The X-ray spectroscopy done by Henry Moseley in 1914 showed a direct dependency between spectral line and effective nuclear charge. This led to the nuclear charge, or atomic number of an element, being used to ascertain its place within the periodic table. With this method, Moseley determined the number of lanthanides and showed the gaps in the atomic number sequence at numbers 43, 61, 72, and 75.[21]
The discovery of the gaps led to an extensive search for the missing elements. In 1914, several people claimed the discovery after Henry Moseley predicted the gap in the periodic table for the then-undiscovered element 72.[22] Georges Urbain asserted that he found element 72 in the rare earth elements in 1907 and published his results on celtium in 1911.[23] Neither the spectra nor the chemical behavior he claimed matched with the element found later, and therefore his claim was turned down after a long-standing controversy.[24] The controversy was partly because the chemists favored the chemical techniques which led to the discovery of celtium, while the physicists relied on the use of the new X-ray spectroscopy method that proved that the substances discovered by Urbain did not contain element 72.[24] In 1921, Charles R. Bury[25][26] suggested that element 72 should resemble zirconium and therefore was not part of the rare earth elements group. By early 1923, Niels Bohr and others agreed with Bury.[27][28] These suggestions were based on Bohr's theories of the atom which were identical to chemist Charles Bury,[25] the X-ray spectroscopy of Moseley, and the chemical arguments of Friedrich Paneth.[29][30]
Encouraged by these suggestions and by the reappearance in 1922 of Urbain's claims that element 72 was a rare earth element discovered in 1911, Dirk Coster and Georg von Hevesy were motivated to search for the new element in zirconium ores.[31] Hafnium was discovered by the two in 1923 in Copenhagen, Denmark, validating the original 1869 prediction of Mendeleev.[32][33] It was ultimately found in zircon in Norway through X-ray spectroscopy analysis.[34] The place where the discovery took place led to the element being named for the Latin name for "Copenhagen", Hafnia, the home town of Niels Bohr.[35] Today, the Faculty of Science of the University of Copenhagen uses in its seal a stylized image of the hafnium atom.[36]
Hafnium was separated from zirconium through repeated recrystallization of the double ammonium or potassium fluorides by Valdemar Thal Jantzen and von Hevesey.[13] Anton Eduard van Arkel and Jan Hendrik de Boer were the first to prepare metallic hafnium by passing hafnium tetraiodide vapor over a heated tungsten filament in 1924.[14][18] This process for differential purification of zirconium and hafnium is still in use today.[1]
In 1923, six predicted elements were still missing from the periodic table: 43 (technetium), 61 (promethium), 85 (astatine), and 87 (francium) are radioactive elements and are only present in trace amounts in the environment,[37] thus making elements 75 (rhenium) and 72 (hafnium) the last two unknown non-radioactive elements.
التطبيقات
Most of the hafnium produced is used in the manufacture of control rods for nuclear reactors.[15]
Several details contribute to the fact that there are only a few technical uses for hafnium: First, the close similarity between hafnium and zirconium makes it possible to use the more abundant zirconium for most applications; second, hafnium was first available as pure metal after the use in the nuclear industry for hafnium-free zirconium in the late 1950s. Furthermore, the low abundance and difficult separation techniques necessary make it a scarce commodity.[1] When the demand for hafnium-free zirconium dropped following the Fukushima disaster, the price of hafnium increased sharply from around $500–600/kg in 2014 to around $1000/kg in 2015.[38]
المفاعلات النووية
يستخدم الهفنيوم مع الزركونيوم في المفاعلات النووية لتغطية أقطاب اليورانيوم.
The nuclei of several hafnium isotopes can each absorb multiple neutrons. This makes hafnium a good material for use in the control rods for nuclear reactors. Its neutron capture cross section (Capture Resonance Integral Io ≈ 2000 barns)[39] is about 600 times that of zirconium (other elements that are good neutron-absorbers for control rods are cadmium and boron). Excellent mechanical properties and exceptional corrosion-resistance properties allow its use in the harsh environment of pressurized water reactors.[15] The German research reactor FRM II uses hafnium as a neutron absorber.[40] It is also common in military reactors, particularly in US naval reactors,[41] but seldom found in civilian ones, the first core of the Shippingport Atomic Power Station (a conversion of a naval reactor) being a notable exception.[42]
السبائك
Hafnium is used in alloys with iron, titanium, niobium, tantalum, and other metals. An alloy used for liquid-rocket thruster nozzles, for example the main engine of the Apollo Lunar Modules, is C103 which consists of 89% niobium, 10% hafnium and 1% titanium.[43]
Small additions of hafnium increase the adherence of protective oxide scales on nickel-based alloys. It improves thereby the corrosion resistance especially under cyclic temperature conditions that tend to break oxide scales by inducing thermal stresses between the bulk material and the oxide layer.[44][45][46]
المعالجات الدقيقة
Hafnium-based compounds are employed in gate insulators in the 45 nm generation of integrated circuits from Intel, IBM and others.[47][48] Hafnium oxide-based compounds are practical high-k dielectrics, allowing reduction of the gate leakage current which improves performance at such scales.[49][50]
Isotope geochemistry
Isotopes of hafnium and lutetium (along with ytterbium) are also used in isotope geochemistry and geochronological applications, in lutetium-hafnium dating. It is often used as a tracer of isotopic evolution of Earth's mantle through time.[51] This is because 176Lu decays to 176Hf with a half-life of approximately 37 billion years.[52][53][54]
In most geologic materials, zircon is the dominant host of hafnium (>10,000 ppm) and is often the focus of hafnium studies in geology.[55] Hafnium is readily substituted into the zircon crystal lattice, and is therefore very resistant to hafnium mobility and contamination. Zircon also has an extremely low Lu/Hf ratio, making any correction for initial lutetium minimal. Although the Lu/Hf system can be used to calculate a "model age", i.e. the time at which it was derived from a given isotopic reservoir such as the depleted mantle, these "ages" do not carry the same geologic significance as do other geochronological techniques as the results often yield isotopic mixtures and thus provide an average age of the material from which it was derived.
Garnet is another mineral that contains appreciable amounts of hafnium to act as a geochronometer. The high and variable Lu/Hf ratios found in garnet make it useful for dating metamorphic events.[56]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
استخدامات أخرى
Due to its heat resistance and its affinity to oxygen and nitrogen, hafnium is a good scavenger for oxygen and nitrogen in gas-filled and incandescent lamps. Hafnium is also used as the electrode in plasma cutting because of its ability to shed electrons into air.[57]
The high energy content of 178m2Hf was the concern of a DARPA-funded program in the US. This program eventually concluded that using the above-mentioned 178m2Hf nuclear isomer of hafnium to construct high-yield weapons with X-ray triggering mechanisms—an application of induced gamma emission—was infeasible because of its expense. See hafnium controversy.
Hafnium metallocene compounds can be prepared from hafnium tetrachloride and various cyclopentadiene-type ligand species. Perhaps the simplest hafnium metallocene is hafnocene dichloride. Hafnium metallocenes are part of a large collection of Group 4 transition metal metallocene catalysts [58] that are used worldwide in the production of polyolefin resins like polyethylene and polypropylene.
A pyridyl-amidohafnium catalyst can be used for the controlled iso-selective polymerization of propylene which can then be combined with polyethylene to make a much tougher recycled plastic.[59]
Hafnium diselenide is studied in spintronics thanks to its charge density wave and superconductivity.[60]
محاذير
Care needs to be taken when machining hafnium because it is pyrophoric—fine particles can spontaneously combust when exposed to air. Compounds that contain this metal are rarely encountered by most people. The pure metal is not considered toxic, but hafnium compounds should be handled as if they were toxic because the ionic forms of metals are normally at greatest risk for toxicity, and limited animal testing has been done for hafnium compounds.[61]
People can be exposed to hafnium in the workplace by breathing it in, swallowing it, skin contact, and eye contact. The Occupational Safety and Health Administration (OSHA) has set the legal limit (permissible exposure limit) for exposure to hafnium and hafnium compounds in the workplace as TWA 0.5 mg/m3 over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set the same recommended exposure limit (REL). At levels of 50 mg/m3, hafnium is immediately dangerous to life and health.[62]
السمية
معدن الهفنيوم لا يسبب أية اضطرابات، لكن مركباته يشار إليها على أنها سامة وبالرغم من ذلك فإن عوامل الخطورة محدودة. هذا المعدن غير قابل للذوبان في الماء، أو المحاليل الملحية (السلاين) أو في المواد الكيميائية بالجسد. التعرض لهذا المعدن من خلال التنفس أو التلامس عن طريق العين أو الجلد أو الطعام. التعرض المفرط لمعدن الهفنيوم أو مركباته يسبب استثارة معتدلة للأعين أو الجلد أو الغشاء المخاطى للحيوانات المعملية. لا توجد أية أعراض أو علامات للتعرض المزمن للهفنيوم للإنسان، أو حدوث حالات للتسمم إذا تم تناوله من خلال الأطعمة.[63]:
انظر أيضاً
المصادر
- ^ أ ب ت ث ج ح Schemel, J. H. (1977). ASTM Manual on Zirconium and Hafnium. Vol. STP 639. Philadelphia: ASTM. ASTM Committee B10 on Reactive and Refractory Metal and Alooys. pp. 1–5. ISBN 978-0-8031-0505-8.
- ^ O'Hara, Andrew; Demkov, Alexander A. (2014). "Oxygen and nitrogen diffusion in α-hafnium from first principles". Applied Physics Letters. 104 (21): 211909. Bibcode:2014ApPhL.104u1909O. doi:10.1063/1.4880657.
- ^ أ ب Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). Lehrbuch der Anorganischen Chemie (in الألمانية) (91–100 ed.). Walter de Gruyter. pp. 1056–1057. doi:10.1515/9783110206845. ISBN 978-3-11-007511-3.
- ^ أ ب Barbalace, Kenneth L. "Periodic Table of Elements: Hf – Hafnium". environmentalchemistry.com. J.K. Barbalace Inc. Retrieved 2021-11-12.
- ^ أ ب قالب:NUBASE 2016
- ^ Caracciolo, V.; Nagorny, S.; Belli, P.; et al. (2020). "Search for α decay of naturally occurring Hf-nuclides using a Cs2HfCl6 scintillator". Nuclear Physics A. 1002 (121941): 121941. arXiv:2005.01373. Bibcode:2020NuPhA100221941C. doi:10.1016/j.nuclphysa.2020.121941. S2CID 218487451.
- ^ Kleine T, Walker RJ (August 2017). "Tungsten Isotopes in Planets". Annual Review of Earth and Planetary Sciences. 45 (1): 389–417. Bibcode:2017AREPS..45..389K. doi:10.1146/annurev-earth-063016-020037. PMC 6398955. PMID 30842690.
- ^ Deer, William Alexander; Howie, Robert Andrew; Zussmann, Jack (1982). The Rock-Forming Minerals: Orthosilicates. Vol. 1A. Longman Group Limited. pp. 418–442. ISBN 978-0-582-46526-8.
- ^ Lee, O. Ivan (1928). "The Mineralogy of Hafnium". Chemical Reviews. 5 (1): 17–37. doi:10.1021/cr60017a002.
- ^ Chalmers, Ian (June 2007). "The Dubbo Zirconia Project" (PDF). Alkane Resources Limited. Archived from the original (PDF) on 2008-02-28. Retrieved 2008-09-10.
- ^ Gambogi, Joseph (2010). "2008 Minerals Yearbook: Zirconium and Hafnium". United States Geological Survey. Retrieved 2021-11-11.
- ^ Larsen, Edwin M.; Fernelius, W. Conard; Quill, Laurence (1943). "Concentration of Hafnium. Preparation of Hafnium-Free Zirconia". Ind. Eng. Chem. Anal. Ed. 15 (8): 512–515. doi:10.1021/i560120a015.
- ^ أ ب van Arkel, A. E.; de Boer, J. H. (1924). "Die Trennung von Zirkonium und Hafnium durch Kristallisation ihrer Ammoniumdoppelfluoride (The separation of zirconium and hafnium by crystallization of their double ammonium fluorides)". Zeitschrift für Anorganische und Allgemeine Chemie (in الألمانية). 141: 284–288. doi:10.1002/zaac.19241410117.
- ^ أ ب van Arkel, A. E.; de Boer, J. H. (1924-12-23). "Die Trennung des Zirkoniums von anderen Metallen, einschließlich Hafnium, durch fraktionierte Distillation" [The separation of zirconium from other metals, including hafnium, by fractional distillation]. Zeitschrift für Anorganische und Allgemeine Chemie (in الألمانية). 141 (1): 289–296. doi:10.1002/zaac.19241410118.
- ^ أ ب ت Hedrick, James B. "Hafnium" (PDF). United States Geological Survey. Retrieved 2008-09-10.
- ^ Griffith, Robert F. (1952). "Zirconium and hafnium". Minerals yearbook metals and minerals (except fuels). The first production plants Bureau of Mines. pp. 1162–1171.
- ^ Gilbert, H. L.; Barr, M. M. (1955). "Preliminary Investigation of Hafnium Metal by the Kroll Process". Journal of the Electrochemical Society. 102 (5): 243. doi:10.1149/1.2430037.
- ^ أ ب van Arkel, A. E.; de Boer, J. H. (1925). "Darstellung von reinem Titanium-, Zirkonium-, Hafnium- und Thoriummetall (Production of pure titanium, zirconium, hafnium and Thorium metal)". Zeitschrift für Anorganische und Allgemeine Chemie (in الألمانية). 148: 345–350. doi:10.1002/zaac.19251480133.
- ^ موفق شخاشيرو. "الهفنيوم". الموسوعة العربية. Retrieved 2012-03-26.
- ^ Kaji, Masanori (2002). "D. I. Mendeleev's concept of chemical elements and The Principles of Chemistry" (PDF). Bulletin for the History of Chemistry. 27: 4. Archived from the original (PDF) on 2008-12-17. Retrieved 2008-08-20.
- ^ Heilbron, John L. (1966). "The Work of H. G. J. Moseley". Isis. 57 (3): 336. doi:10.1086/350143. S2CID 144765815.
- ^ Heimann, P. M. (1967). "Moseley and celtium: The search for a missing element". Annals of Science. 23 (4): 249–260. doi:10.1080/00033796700203306.
- ^ Urbain, M. G. (1911). "Sur un nouvel élément qui accompagne le lutécium et le scandium dans les terres de la gadolinite: le celtium (On a new element that accompanies lutetium and scandium in gadolinite: celtium)". Comptes Rendus (in الفرنسية): 141. Retrieved 2008-09-10.
- ^ أ ب Mel'nikov, V. P. (1982). "Some Details in the Prehistory of the Discovery of Element 72". Centaurus. 26 (3): 317–322. Bibcode:1982Cent...26..317M. doi:10.1111/j.1600-0498.1982.tb00667.x.
- ^ أ ب Kragh, Helge. “Niels Bohr’s Second Atomic Theory.” Historical Studies in the Physical Sciences, vol. 10, University of California Press, 1979, pp. 123–86, https://doi.org/10.2307/27757389.
- ^ Bury, Charles R. (1921). "Langmuir's Theory of the Arrangement of Electrons in Atoms and Molecules". J. Am. Chem. Soc. 43 (7): 1602–1609. doi:10.1021/ja01440a023.
- ^ Bohr, Niels (June 2008). The Theory of Spectra and Atomic Constitution: Three Essays. p. 114. ISBN 978-1-4365-0368-6.
- ^ Niels Bohr (11 December 1922). "Nobel Lecture: The Structure of the Atom" (PDF). Retrieved 25 March 2021.
- ^ Paneth, F. A. (1922). "Das periodische System (The periodic system)". Ergebnisse der Exakten Naturwissenschaften 1 (in الألمانية). p. 362.
- ^ Fernelius, W. C. (1982). "Hafnium" (PDF). Journal of Chemical Education. 59 (3): 242. Bibcode:1982JChEd..59..242F. doi:10.1021/ed059p242. Archived from the original (PDF) on 2020-03-15. Retrieved 2009-09-03.
- ^ Urbain, M. G. (1922). "Sur les séries L du lutécium et de l'ytterbium et sur l'identification d'un celtium avec l'élément de nombre atomique 72" [The L series from lutetium to ytterbium and the identification of element 72 celtium]. Comptes Rendus (in الفرنسية). 174: 1347. Retrieved 2008-10-30.
- ^ Coster, D.; Hevesy, G. (1923). "On the Missing Element of Atomic Number 72". Nature. 111 (2777): 79. Bibcode:1923Natur.111...79C. doi:10.1038/111079a0.
- ^ Hevesy, G. (1925). "The Discovery and Properties of Hafnium". Chemical Reviews. 2: 1–41. doi:10.1021/cr60005a001.
- ^ von Hevesy, Georg (1923). "Über die Auffindung des Hafniums und den gegenwärtigen Stand unserer Kenntnisse von diesem Element". Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 56 (7): 1503–1516. doi:10.1002/cber.19230560702. S2CID 96017606.
- ^ Scerri, Eric R. (1994). "Prediction of the nature of hafnium from chemistry, Bohr's theory and quantum theory". Annals of Science. 51 (2): 137–150. doi:10.1080/00033799400200161.
- ^ "University Life 2005" (pdf). University of Copenghagen. p. 43. Retrieved 2016-11-19.
- ^ Curtis, David; Fabryka-Martin, June; Dixon, Pauland; Cramer, Jan (1999). "Nature's uncommon elements: plutonium and technetium". Geochimica et Cosmochimica Acta. 63 (2): 275–285. Bibcode:1999GeCoA..63..275C. doi:10.1016/S0016-7037(98)00282-8.
- ^ Albrecht, Bodo (2015-03-11). "Weak Zirconium Demand Depleting Hafnium Stock Piles". Tech Metals Insider. KITCO. Retrieved 4 March 2018.
- ^ https://www.oecd-nea.org/dbdata/nds_jefreports/jefreport-23/supp/jefdoc/jefdoc-1077.pdf Noguère G., Courcelle A., Palau J.M., Siegler P. (2005) Low-neutron-energy cross sections of the hafnium isotopes.
- ^ "Forschungsreaktor München II (FRM-II): Standort und Sicherheitskonzept" (PDF). Strahlenschutzkommission. 1996-02-07. Archived from the original (PDF) on October 20, 2007. Retrieved 2008-09-22.
- ^ J. H. Schemel (1977). ASTM Manual on Zirconium and Hafnium. ASTM International. p. 21. ISBN 978-0-8031-0505-8.
- ^ C.W. Forsberg; K. Takase & N. Nakatsuka (2011). "Water Reactor". In Xing L. Yan & Ryutaro Hino (eds.). Nuclear Hydrogen Production Handbook. CRC Press. p. 192. ISBN 978-1-4398-1084-2.
- ^ Hebda, John (2001). "Niobium alloys and high Temperature Applications" (PDF). CBMM. Archived from the original (PDF) on 2008-12-17. Retrieved 2008-09-04.
- ^ Maslenkov, S. B.; Burova, N. N.; Khangulov, V. V. (1980). "Effect of hafnium on the structure and properties of nickel alloys". Metal Science and Heat Treatment. 22 (4): 283–285. Bibcode:1980MSHT...22..283M. doi:10.1007/BF00779883. S2CID 135595958.
- ^ Beglov, V. M.; Pisarev, B. K.; Reznikova, G. G. (1992). "Effect of boron and hafnium on the corrosion resistance of high-temperature nickel alloys". Metal Science and Heat Treatment. 34 (4): 251–254. Bibcode:1992MSHT...34..251B. doi:10.1007/BF00702544. S2CID 135844921.
- ^ Voitovich, R. F.; Golovko, É. I. (1975). "Oxidation of hafnium alloys with nickel". Metal Science and Heat Treatment. 17 (3): 207–209. Bibcode:1975MSHT...17..207V. doi:10.1007/BF00663680. S2CID 137073174.
- ^ {{{1}}} patent {{{2}}}
- ^ Markoff, John (2007-01-27). "Intel Says Chips Will Run Faster, Using Less Power". New York Times. Retrieved 2008-09-10.
- ^ Fulton III, Scott M. (January 27, 2007). "Intel Reinvents the Transistor". BetaNews. Retrieved 2007-01-27.
- ^ Robertson, Jordan (January 27, 2007). "Intel, IBM reveal transistor overhaul". The Associated Press. Retrieved 2008-09-10.
- ^ Patchett, P. Jonathan (January 1983). "Importance of the Lu-Hf isotopic system in studies of planetary chronology and chemical evolution". Geochimica et Cosmochimica Acta. 47 (1): 81–91. Bibcode:1983GeCoA..47...81P. doi:10.1016/0016-7037(83)90092-3.
- ^ Söderlund, Ulf; Patchett, P. Jonathan; Vervoort, Jeffrey D.; Isachsen, Clark E. (March 2004). "The 176Lu decay constant determined by Lu–Hf and U–Pb isotope systematics of Precambrian mafic intrusions". Earth and Planetary Science Letters. 219 (3–4): 311–324. Bibcode:2004E&PSL.219..311S. doi:10.1016/S0012-821X(04)00012-3.
- ^ Blichert-Toft, Janne; Albarède, Francis (April 1997). "The Lu-Hf isotope geochemistry of chondrites and the evolution of the mantle-crust system". Earth and Planetary Science Letters. 148 (1–2): 243–258. Bibcode:1997E&PSL.148..243B. doi:10.1016/S0012-821X(97)00040-X.
- ^ Patchett, P. J.; Tatsumoto, M. (11 December 1980). "Lu–Hf total-rock isochron for the eucrite meteorites". Nature. 288 (5791): 571–574. Bibcode:1980Natur.288..571P. doi:10.1038/288571a0. S2CID 4284487.
- ^ Kinny, P. D. (1 January 2003). "Lu-Hf and Sm-Nd isotope systems in zircon". Reviews in Mineralogy and Geochemistry. 53 (1): 327–341. Bibcode:2003RvMG...53..327K. doi:10.2113/0530327.
- ^ Albarède, F.; Duchêne, S.; Blichert-Toft, J.; Luais, B.; Télouk, P.; Lardeaux, J.-M. (5 June 1997). "The Lu–Hf dating of garnets and the ages of the Alpine high-pressure metamorphism". Nature. 387 (6633): 586–589. Bibcode:1997Natur.387..586D. doi:10.1038/42446. S2CID 4260388.
- ^ Ramakrishnany, S.; Rogozinski, M. W. (1997). "Properties of electric arc plasma for metal cutting". Journal of Physics D: Applied Physics. 30 (4): 636–644. Bibcode:1997JPhD...30..636R. doi:10.1088/0022-3727/30/4/019. S2CID 250746818.
- ^ g. Alt, Helmut; Samuel, Edmond (1998). "Fluorenyl complexes of zirconium and hafnium as catalysts for olefin polymerization". Chem. Soc. Rev. 27 (5): 323–329. doi:10.1039/a827323z.
- ^ Eagan, James (24 Feb 2017). "Combining polyethylene and polypropylene: Enhanced performance with PE/iPP multiblock polymers". Science. 355 (6327): 814–816. Bibcode:2017Sci...355..814E. doi:10.1126/science.aah5744. PMID 28232574. S2CID 206652330.
- ^ Helmholtz Association of German Research Centres (September 7, 2022). "A new road towards spin-polarized currents". Nature Communications. Phys.org. 13 (1): 4147. doi:10.1038/s41467-022-31539-2. PMC 9288546. PMID 35842436.
- ^ "Occupational Safety & Health Administration: Hafnium". U.S. Department of Labor. Archived from the original on 2008-03-13. Retrieved 2008-09-10.
- ^ "CDC - NIOSH Pocket Guide to Chemical Hazards - Hafnium". www.cdc.gov. Retrieved 2015-11-03.
- ^ المعادن الثقيلة.. سموم بيئية، فيدو
المراجع
- هيام بيرقدار، الكيمياء اللاعضوية 2 (مطبعة محمد هاشم الكتبي، 1391هـ/1971م).
- MARY ELVIRA WEEKS, Discovery of the Elements (Mack Printing Company 1956).
وصلات خارجية
- Hafnium at Los Alamos National Laboratory's periodic table of the elements
- Hafnium Technical & Safety Data
- NLM Hazardous Substances Databank – Hafnium, elemental
- Intel Shifts from Silicon to Lift Chip Performance
- Hafnium-based Intel 45nm Process Technology
| الجدول الدوري | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Uub | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
| |||||||||||||||||||||||||||||||||||||||||



![{\displaystyle {\ce {HfCl4 + 2Mg ->[1100^oC] Hf + 2MgCl2}}}](https://www.marefa.org/api/rest_v1/media/math/render/svg/aa72f37e998474c30ff0a68803a831241768a664)
![{\displaystyle {\ce {Hf + 2I2 ->[500^oC] HfI4}}}](https://www.marefa.org/api/rest_v1/media/math/render/svg/ac2cfa9d8a39e3fc014956d17bc17a4d7b22bbed)
![{\displaystyle {\ce {HfI4 ->[1700^oC] Hf + 2I2}}}](https://www.marefa.org/api/rest_v1/media/math/render/svg/f3f0df30a8edc787cb1735a3dc40ac05af6e924f)