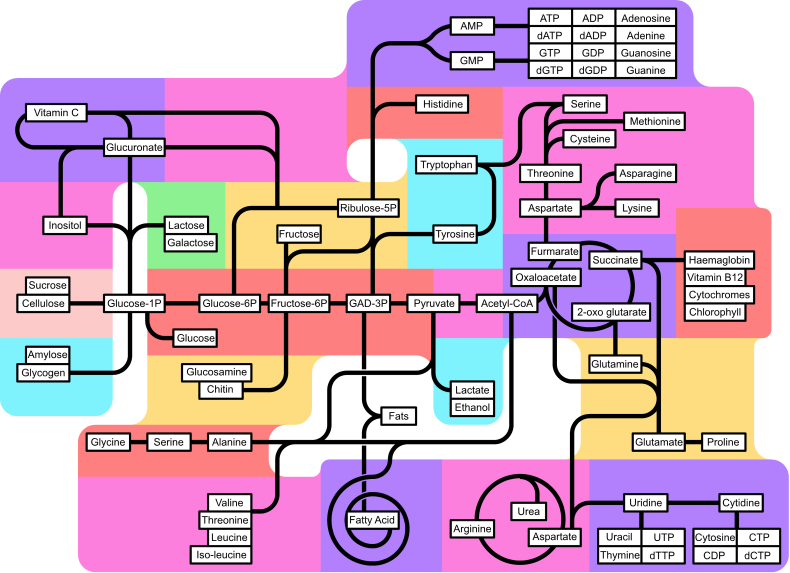
أيض الحمض الدهني
إستقلاب الحمض الدهني بالإنجليزية Fatty acid metabolism ، عملية إستقلاب الدهون تعتبر مصدر هام من مصادر الطاقة للكثير من أعضاء الجسم. قبل أكسدة المواد الدهنية لإنتاج الطاقة ، يتم إستقدام هذه الدهون من مخازننها وتوصيلها إلى الدم على هيئة أحماض دهنية حرة ، ومن الدم يتم توزيعها على الأنسجة المختلفة مثل الكبد و العضلات والتي يتم أكسدة الأحماض الدهنية فيها.
هدم الدهون
توجد الدهون كمادة مخزنة في النباتات الراقية خاصة في البذور. وتحلل الدهون يتم للحصول على الطاقة في صورة أدينوسين ثلاثي الفوسفات او يستخدم في بناء السكريات عن طريق دورة الجلوكوزيت glyoxylate cycle لبناء الجلوكوز . يبدأ تحلل الدهون بتكسير الجزيئات الى جليسرول و أحماض دهنية بمساعدة انزيمات لايبيز يدخل الجليسرول في بناء السكريات او بهدم في هذه السترات.
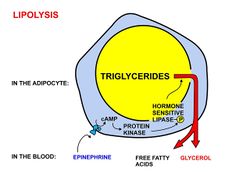
Fatty acids are released, between meals, from the fat depots in adipose tissue, where they are stored as triglycerides, as follows:
- Lipolysis, the removal of the fatty acid chains from the glycerol to which they are bound in their storage form as triglycerides (or fats), is carried out by lipases. These lipases are activated by high epinephrine and glucagon levels in the blood (or norepinephrine secreted by sympathetic nerves in adipose tissue), caused by declining blood glucose levels after meals, which simultaneously lowers the insulin level in the blood.[2]
- Once freed from glycerol, the free fatty acids enter the blood, which transports them, attached to plasma albumin, throughout the body.[3]
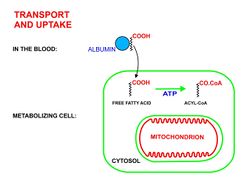
- Long chain free fatty acids enter the metabolizing cells (i.e. most living cells in the body except red blood cells and neurons in the central nervous system) through specific transport proteins, such as the SLC27 family fatty acid transport protein.[4][5] Red blood cells do not contain mitochondria and are therefore incapable of metabolizing fatty acids; the tissues of the central nervous system cannot use fatty acids, despite containing mitochondria, because long chain fatty acids (as opposed to medium chain fatty acids[6][7]) cannot cross the blood brain barrier[8] into the interstitial fluids that bathe these cells.
- Once inside the cell long-chain-fatty-acid—CoA ligase catalyzes the reaction between a fatty acid molecule with ATP (which is broken down to AMP and inorganic pyrophosphate) to give a fatty acyl-adenylate, which then reacts with free coenzyme A to give a fatty acyl-CoA molecule.
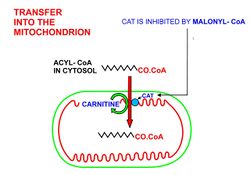
- In order for the acyl-CoA to enter the mitochondrion the carnitine shuttle is used:[9][10][11]
- Acyl-CoA is transferred to the hydroxyl group of carnitine by carnitine palmitoyltransferase I, located on the cytosolic faces of the outer and inner mitochondrial membranes.
- Acyl-carnitine is shuttled inside by a carnitine-acylcarnitine translocase, as a carnitine is shuttled outside.
- Acyl-carnitine is converted back to acyl-CoA by carnitine palmitoyltransferase II, located on the interior face of the inner mitochondrial membrane. The liberated carnitine is shuttled back to the cytosol, as an acyl-CoA is shuttled into the matrix.
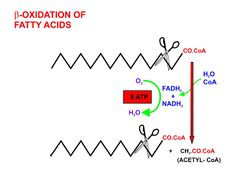
- Beta oxidation, in the mitochondrial matrix, then cuts the long carbon chains of the fatty acids (in the form of acyl-CoA molecules) into a series of two-carbon (acetate) units, which, combined with co-enzyme A, form molecules of acetyl CoA, which condense with oxaloacetate to form citrate at the "beginning" of the citric acid cycle.[12] It is convenient to think of this reaction as marking the "starting point" of the cycle, as this is when fuel - acetyl-CoA - is added to the cycle, which will be dissipated as CO2 and H2O with the release of a substantial quantity of energy captured in the form of ATP, during the course of each turn of the cycle.
- Briefly, the steps in beta oxidation (the initial breakdown of free fatty acids into acetyl-CoA) are as follows:[12]
- Dehydrogenation by acyl-CoA dehydrogenase, yielding 1 FADH2
- Hydration by enoyl-CoA hydratase
- Dehydrogenation by 3-hydroxyacyl-CoA dehydrogenase, yielding 1 NADH + H+
- Cleavage by thiolase, yielding 1 acetyl-CoA and a fatty acid that has now been shortened by 2 carbons (forming a new, shortened acyl-CoA)
- This beta oxidation reaction is repeated until the fatty acid has been completely reduced to acetyl-CoA or, in, the case of fatty acids with odd numbers of carbon atoms, acetyl-CoA and 1 molecule of propionyl-CoA per molecule of fatty acid. Each beta oxidative cut of the acyl-CoA molecule yields 5 ATP molecules.[13][14]
- The acetyl-CoA produced by beta oxidation enters the citric acid cycle in the mitochondrion by combining with oxaloacetate to form citrate. This results in the complete combustion of the acetyl-CoA to CO2 and water. The energy released in this process is captured in the form of 1 GTP and 11 ATP molecules per acetyl-CoA molecule oxidized.[12][9] This is the fate of acetyl-CoA wherever beta oxidation of fatty acids occurs, except under certain circumstances in the liver.
In the liver oxaloacetate can be wholly or partially diverted into the gluconeogenic pathway during fasting, starvation, a low carbohydrate diet, prolonged strenuous exercise, and in uncontrolled type 1 diabetes mellitus. Under these circumstances oxaloacetate is hydrogenated to malate which is then removed from the mitochondrion to be converted into glucose in the cytoplasm of the liver cells, from where it is released into the blood.[9] In the liver, therefore, oxaloacetate is unavailable for condensation with acetyl-CoA when significant gluconeogenesis has been stimulated by low (or absent) insulin and high glucagon concentrations in the blood. Under these circumstances acetyl-CoA is diverted to the formation of acetoacetate and beta-hydroxybutyrate.[9] Acetoacetate, beta-hydroxybutyrate, and their spontaneous breakdown product, acetone, are frequently, but confusingly, known as ketone bodies (as they are not "bodies" at all, but water-soluble chemical substances). The ketone bodies are released by the liver into the blood. All cells with mitochondria can take ketone bodies up from the blood and reconvert them into acetyl-CoA, which can then be used as fuel in their citric acid cycles, as no other tissue can divert its oxaloacetate into the gluconeogenic pathway in the way that this can occur in the liver. Unlike free fatty acids, ketone bodies can cross the blood-brain barrier and are therefore available as fuel for the cells of the central nervous system, acting as a substitute for glucose, on which these cells normally survive.[9] The occurrence of high levels of ketone bodies in the blood during starvation, a low carbohydrate diet, prolonged heavy exercise and uncontrolled type 1 diabetes mellitus is known as ketosis, and, in its extreme form, in out-of-control type 1 diabetes mellitus, as ketoacidosis.
- The glycerol released by lipase action is phosphorylated by glycerol kinase in the liver (the only tissue in which this reaction can occur), and the resulting glycerol 3-phosphate is oxidized to dihydroxyacetone phosphate. The glycolytic enzyme triose phosphate isomerase converts this compound to glyceraldehyde 3-phosphate, which is oxidized via glycolysis, or converted to glucose via gluconeogenesis.
الأحماض الدهنية كمصدر للطاقة
Fatty acids, stored as triglycerides in an organism, are an important source of energy because they are both reduced and anhydrous. The energy yield from a gram of fatty acids is approximately 9 kcal (37 kJ), compared to 4 kcal (17 kJ) for carbohydrates. Since the hydrocarbon portion of fatty acids is hydrophobic, these molecules can be stored in a relatively anhydrous (water-free) environment. Carbohydrates, on the other hand, are more highly hydrated. For example, 1 g of glycogen can bind approximately 2 g of water, which translates to 1.33 kcal/g (4 kcal/3 g). This means that fatty acids can hold more than six times the amount of energy per unit of storage mass. Put another way, if the human body relied on carbohydrates to store energy, then a person would need to carry 31 kg (67.5 lb) of hydrated glycogen to have the energy equivalent to 4.6 kg (10 lb) of fat.[9]
Hibernating animals provide a good example for utilizing fat reserves as fuel. For example, bears hibernate for about 7 months, and, during this entire period, the energy is derived from degradation of fat stores. Migrating birds similarly build up large fat reserves before embarking on their intercontinental journeys.[15]
Thus the young adult human’s fat stores average between about 10–20 kg, but varies greatly depending on age, gender, and individual disposition.[16] By contrast the human body stores only about 400 g of glycogen, of which 300 g is locked inside the skeletal muscles and is unavailable to the body as a whole. The 100 g or so of glycogen stored in the liver is depleted within one day of starvation.[9] Thereafter the glucose that is released into the blood by the liver for general use by the body tissues, has to be synthesized from the glucogenic amino acids and a few other gluconeogenic substrates, which do not include fatty acids.[2] Please note however that lipolysis releases glycerol which can enter the pathway of gluconeogenesis.
الحيوانات والنباتات يخلـِّقون النشويات من كل من الگليسرول والأحماض الدهنية
Fatty acids are broken down to acetyl-CoA by means of beta oxidation inside the mitochondria, whereas fatty acids are synthesized from acetyl-CoA outside the mitochondria, in the cytosol. The two pathways are distinct, not only in where they occur, but also in the reactions that occur, and the substrates that are used. The two pathways are mutually inhibitory, preventing the acetyl-CoA produced by beta-oxidation from entering the synthetic pathway via the acetyl-CoA carboxylase reaction.[2] It can also not be converted to pyruvate as the pyruvate dehydrogenase complex reaction is irreversible.[9] Instead the acetyl-CoA produced by the beta-oxidation of fatty acids condenses with oxaloacetate, to enter the citric acid cycle. During each turn of the cycle, two carbon atoms leave the cycle as CO2 in the decarboxylation reactions catalyzed by isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase. Thus each turn of the citric acid cycle oxidizes an acetyl-CoA unit while regenerating the oxaloacetate molecule with which the acetyl-CoA had originally combined to form citric acid. The decarboxylation reactions occur before malate is formed in the cycle.[2] Only plants possess the enzymes to convert acetyl-CoA into oxaloacetate from which malate can be formed to ultimately be converted to glucose.[2]
However acetyl-CoA can be converted to acetoacetate, which can decarboxylate to acetone (either spontaneously, or by acetoacetate decarboxylase). It can then be further metabolized to isopropanol which is excreted in breath/urine, or by CYP2E1 into hydroxyacetone (acetol). Acetol can be converted to propylene glycol. This converts to formate and acetate (the latter converting to glucose), or pyruvate (by two alternative enzymes), or propionaldehyde, or to L-lactaldehyde then L-lactate (the common lactate isomer).[17][18][19] Another pathway turns acetol to methylglyoxal, then to pyruvate, or to D-lactaldehyde (via S-D-lactoyl-glutathione or otherwise) then D-lactate.[18][20][21] D-lactate metabolism (to glucose) is slow or impaired in humans, so most of the D-lactate is excreted in the urine; thus D-lactate derived from acetone can contribute significantly to the metabolic acidosis associated with ketosis or isopropanol intoxication.[18] L-Lactate can complete the net conversion of fatty acids into glucose. The first experiment to show conversion of acetone to glucose was carried out in 1951. This, and further experiments used carbon isotopic labelling.[19] Up to 11% of the glucose can be derived from acetone during starvation in humans.[19]
The glycerol released into the blood during the lipolysis of triglycerides in adipose tissue can only be taken up by the liver. Here it is converted into glycerol 3-phosphate by the action of glycerol kinase which hydrolyzes one molecule of ATP per glycerol molecule which is phosphorylated. Glycerol 3-phosphate is then oxidized to dihydroxyacetone phosphate, which is, in turn, converted into glyceraldehyde 3-phosphate by the enzyme triose phosphate isomerase. From here the three carbon atoms of the original glycerol can be oxidized via glycolysis, or converted to glucose via gluconeogenesis.[9]
ميكانيكية أيض الدهون
يحتوي الكبد على مخزون من الدهون المتعادلة والفسفورية ، كما أنه يستقبل بشكل دائم دهونا متعادلة من الغذاء ، ومن الدهون المخزنة في الجسم بهدف أكسدتها ، وسواء أكانت المادة التي أكدستها الكبد دهونا متعادلة أو فوسفورية ، فإن الجزء الأساسي في هذه العملية يتمثل في أكسدة الأحماض الدهنية طويلة السلسلة في الميتوكندريا. أما جزئ الجلسرين فيتفاعل مع جزي أدينوسين ثلاثي الفوسفات لإنتاج فوسفات الجلسرين التي تتم أكسدتها إلى جليرسيرالدهيد – 3 – فوسفات ، وهذا الجزئ إما أن يتحول إلى جليكوجين من خلال المسار العكسي لعملية تحلل الجلوكوز ، وإما أن يتحول إلى حمض البيرفيك. وتتم أيضا أكسدة الأحماض الدهنية في العضلات ، ففي العضلات القلبية تمثل الأحماض الدهنية بالفعل مصدرا معما للطاقة و التنفس ، كما تؤكسد عضلة الحجاب الحاجز الأحماض الدهنية ، أما إذا وجد الجلوكوز فإن هذه العضلة تفضله على الأحماض الدهني. وطبقا لنظرية أكسدة الأحماض الدهنية من نوع بيتا ، فإن سلاسل الأحماض الدهنية تتم أكسدتها: للتخلص من ذرتي كربون في كل مرة ويتم ذلك بمهاجمة ذرة الكربون في الموقع بيتا بالنسبة إلى مجموعة الكربوكسيل بحيث يتحول الحمض الدهني إلى الحمض الكيتوني المقابل ، أما ذرتا الكربون الطرفيتنات فيتم التخلص منهما على هيئة حمض خليك ، ثم يتم عمل مجموعة كربوكسيل في موضع المجموعة الكيتونية ، وبذلك يتكون حمض دهني جديد ينقص عن الحمض الأصلي بمقدار ذرتي كربون ، ويلي ذلك مهاجمة ذرة كربون مرة أخرى في الموقع بيتا لتتكرر الخطوات السابقة ، وينتج حمض دهني جديد يقل ذرتي كربون عن الحمض الأصلي. وبهذه الطريقة يتم تكسير الحمض الدهني ، بإزالة ذرتي الكربون في كل مرة ينتج في المرحلة النهائية جزئ يسمى حمض الخليك الخللي ، الذي يسمى أيضا حمض بيتا – كيتوبيوتيريك. ولكي يتم أكسدة الاحماض الدهنية ، يلزم أولا تنشيطها عن طريق تكوين مشتقات المرافق الإنزيمي أ.
الأجسام الكيتونية
يتحد المرافق الإنزيمي الخللي الذي لا يستخدم في تخليق الكوليسترول أو في تخليق الأحماض الدهنية أوالمشتقات الخللية بجزئ الأوكسالوأسيتات لتتم أكسدته إلى ثاني أكسيد الكربون و الماء في دورة كربس. وبينما تتم إستعادة بعض الأوكسالوأسيتات من خلال دورة كربس ، فإن هذه الكمية قد يتم تدعيمها وزيادتها عن طريق إضافة ثاني أكسيد الكربون إلى حمض البيروفيك المتحصل عليه من أيض الكربوهيدرات في خلايا الكبد.
وعلي ذلك فإن إنخفاض أيض المواد الكربوهيدراتية من جراء مرض السكر ينتج عنه هاتان النتيجتان:
- زيادة أكسدة الدهون للحصول على الطاقة كمصدر بديل للمواد النشوية ، والنتيجة النهائية هي زيادة إنتاج المرافق الإنزيمي أ.
- نقص في إنتاج الأوكسالوأسيتات لنقص إنتاج حمض البيروفيك.
وظائف واستخدامات أخرى للأحماض الدهنية
الإشارات داخل الخلية
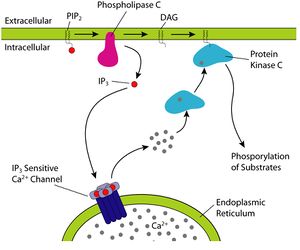
Fatty acids are an integral part of the phospholipids that make up the bulk of the plasma membranes, or cell membranes, of cells. These phospholipids can be cleaved into diacylglycerol (DAG) and inositol trisphosphate (IP3) through hydrolysis of the phospholipid, phosphatidylinositol 4,5-bisphosphate (PIP2), by the cell membrane bound enzyme phospholipase C (PLC).[22]
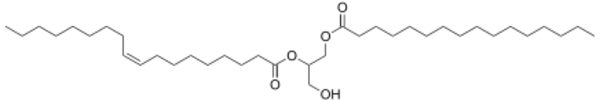
An example of a diacyl-glycerol shown on the right. This DAG is 1-palmitoyl-2-oleoyl-glycerol, which contains side-chains derived from palmitic acid and oleic acid. Diacylglycerols can also have many other combinations of fatty acids attached at either the C-1 and C-2 positions or the C-1 and C-3 positions of the glycerol molecule. 1,2 disubstituted glycerols are always chiral, 1,3 disubstituted glycerols are chiral if the substituents are different from each other.
Eicosanoid paracrine hormones
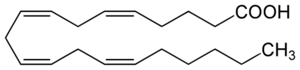
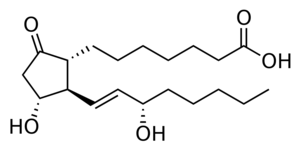
The prostaglandins are a group of physiologically active lipid compounds having diverse hormone-like effects in animals. Prostaglandins have been found in almost every tissue in humans and other animals. They are enzymatically derived from arachidonic acid a 20-carbon polyunsaturated fatty acid. Every prostaglandin therefore contains 20 carbon atoms, including a 5-carbon ring. They are a subclass of eicosanoids and form the prostanoid class of fatty acid derivatives.[23]
المصادر الغذائية للأحماض الدهنية وهضمهم وامتصاصهم ونقلهم في الدم وتخزينهم
A significant proportion of the fatty acids in the body are obtained from the diet, in the form of triglycerides of either animal or plant origin. The fatty acids in the fats obtained from land animals tend to be saturated, whereas the fatty acids in the triglycerides of fish and plants are often polyunsaturated and therefore present as oils.
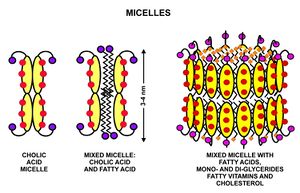
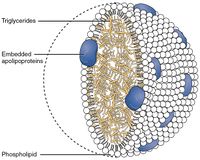
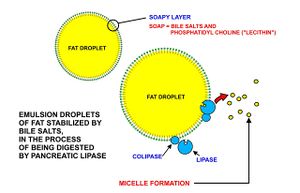
These triglycerides, cannot be absorbed by the intestine.[24] They are broken down into mono- and di-glycerides plus free fatty acids (but no free glycerol) by pancreatic lipase, which forms a 1:1 complex with a protein called colipase (also a constituent of pancreatic juice), which is necessary for its activity. The activated complex can work only at a water-fat interface. Therefore, it is essential that fats are first emulsified by bile salts for optimal activity of these enzymes.[25] The digestion products consisting of a mixture of tri-, di- and monoglycerides and free fatty acids, which, together with the other fat soluble contents of the diet (e.g. the fat soluble vitamins and cholesterol) and bile salts form mixed micelles, in the watery duodenal contents (see diagrams on the right).[24][26]
تخليق الحمض الدهني
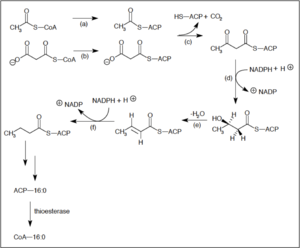
Much like beta-oxidation, straight-chain fatty acid synthesis occurs via the six recurring reactions shown below, until the 16-carbon palmitic acid is produced.[27][28]
بناء وتكوين الدهن يتكون جزى الدهن بتكثيف جزى واحد من كحول الجليسرول الثلاثى الايدروكسيل مع ثلاثة جزيئات من نفس الحمض الدهنى أو احماض دهنية مختلفة. ويشتق الجليسرول و الحمض الدهنى من الكربوهيدرات اثناء التنفس.
توجد الأحماض الدهنية فقط كأثار في الأنسجة النباتية السليمة ويبدو ذلك لاستغلال الأحماض الدهنية في بناء الدهون بنفس السرعة التى تبنى بها الأحماض تقريبا. وتبنى الأحماض الدهنية من استيل كوإنزيم الذى يتكون اثناء عملية التنفس. وينتج من التفاعل الأحماض الدهنية وهى ليست منفردة بل متحدة مع كوإنزيم لتكون مرافقة الانزيمى A للحمض الدهنى والذى يتحد مع الجليسرول ليكون الدهن.
| الخطوة | الإنزيم | التفاعل | الوصف |
|---|---|---|---|
| (a) | Acetyl CoA:ACP transacylase | Activates acetyl CoA for reaction with malonyl-ACP | |
| (b) | Malonyl CoA:ACP transacylase | Activates malonyl CoA for reaction with acetyl-ACP | |
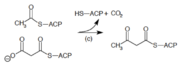
| (c) | 3-ketoacyl-ACP synthase | Reacts priming acetyl-ACP with chain-extending malonyl-ACP. | |
| (d) | 3-ketoacyl-ACP reductase | Reduces the carbon 3 ketone to a hydroxyl group | |
| (e) | 3-Hydroxyacyl ACP dehydrase | Removes water | |
| (f) | Enoyl-ACP reductase | Reduces the C2-C3 double bond. |
Abbreviations: ACP – Acyl carrier protein, CoA – Coenzyme A, NADP – Nicotinamide adenine dinucleotide phosphate.
المنتجات النهائية للتحلل السكري تُستخدم في تحويل النشويات إلى أحماض دهنية
In humans, fatty acids are formed from carbohydrates predominantly in the liver and adipose tissue, as well as in the mammary glands during lactation. The cells of the central nervous system probably also make most of the fatty acids needed for the phospholipids of their extensive membranes from glucose, as blood-born fatty acids cannot cross the blood brain barrier to reach these cells.[29] However, how the essential fatty acids, which mammals cannot synthesize themselves, but are nevertheless important components of cell membranes (and other functions described above) reach them is unknown.
تنظيم تخليق الأحماض الدهنية
Acetyl-CoA is formed into malonyl-CoA by acetyl-CoA carboxylase, at which point malonyl-CoA is destined to feed into the fatty acid synthesis pathway. Acetyl-CoA carboxylase is the point of regulation in saturated straight-chain fatty acid synthesis, and is subject to both phosphorylation and allosteric regulation. Regulation by phosphorylation occurs mostly in mammals, while allosteric regulation occurs in most organisms. Allosteric control occurs as feedback inhibition by palmitoyl-CoA and activation by citrate. When there are high levels of palmitoyl-CoA, the final product of saturated fatty acid synthesis, it allosterically inactivates acetyl-CoA carboxylase to prevent a build-up of fatty acids in cells. Citrate acts to activate acetyl-CoA carboxylase under high levels, because high levels indicate that there is enough acetyl-CoA to feed into the Krebs cycle and produce energy.[30]
High plasma levels of insulin in the blood plasma (e.g. after meals) cause the dephosphorylation of acetyl-CoA carboxylase, thus promoting the formation of malonyl-CoA from acetyl-CoA, and consequently the conversion of carbohydrates into fatty acids, while epinephrine and glucagon (released into the blood during starvation and exercise) cause the phosphorylation of this enzyme, inhibiting lipogenesis in favor of fatty acid oxidation via beta-oxidation.[29][31]
الاضطرابات
Disorders of fatty acid metabolism can be described in terms of, for example, hypertriglyceridemia (too high level of triglycerides), or other types of hyperlipidemia. These may be familial or acquired.
Familial types of disorders of fatty acid metabolism are generally classified as inborn errors of lipid metabolism. These disorders may be described as fatty oxidation disorders or as a lipid storage disorders, and are any one of several inborn errors of metabolism that result from enzyme defects affecting the ability of the body to oxidize fatty acids in order to produce energy within muscles, liver, and other cell types.
وصلات خارجية
انظر أيضا
الهامش
- ^ Zechner R, Strauss JG, Haemmerle G, Lass A, Zimmermann R (2005). "Lipolysis: pathway under construction". Curr. Opin. Lipidol. 16 (3): 333–40. PMID 15891395.
- ^ أ ب ت ث ج خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةstryer1 - ^ Mobilization and cellular uptake of stored fats (triacylglycerols) (with animation)
- ^ Stahl, Andreas (1 February 2004). "A current review of fatty acid transport proteins (SLC27)". Pflügers Archiv: European Journal of Physiology. 447 (5): 722–727. doi:10.1007/s00424-003-1106-z. PMID 12856180. Retrieved 2 March 2015.
- ^ Anderson, Courtney M.; Stahl, Andreas (April 2013). "SLC27 fatty acid transport proteins". Molecular Aspects of Medicine. 34 (2–3): 516–528. doi:10.1016/j.mam.2012.07.010. PMC 3602789.
- ^ Ebert, D.; Haller, RG.; Walton, ME. (Jul 2003). "Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy". J Neurosci. 23 (13): 5928–35. PMID 12843297.
- ^ Marin-Valencia, I.; Good, LB.; Ma, Q.; Malloy, CR.; Pascual, JM. (Feb 2013). "Heptanoate as a neural fuel: energetic and neurotransmitter precursors in normal and glucose transporter I-deficient (G1D) brain". J Cereb Blood Flow Metab. 33 (2): 175–82. doi:10.1038/jcbfm.2012.151. PMC 3564188. PMID 23072752.
- ^ Stryer, Lubert (1995). "Fatty acid metabolism.". In: Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. pp. 770–771. ISBN 0 7167 2009 4.
- ^ أ ب ت ث ج ح خ د ذ Stryer, Lubert (1995). Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. pp. 510–515, 581–613, 775–778. ISBN 0 7167 2009 4.
- ^ Activation and transportation of fatty acids to the mitochondria via the carnitine shuttle (with animation)
- ^ Vivo, Darryl C.; Bohan, Timothy P.; Coulter, David L.; Dreifuss, Fritz E.; Greenwood, Robert S.; Nordli, Douglas R.; Shields, W. Donald; Stafstrom, Carl E.; Tein, Ingrid (1998). "l-Carnitine Supplementation in Childhood Epilepsy: Current Perspectives". Epilepsia. 39 (11): 1216–1225. doi:10.1111/j.1528-1157.1998.tb01315.x. ISSN 0013-9580.
- ^ أ ب ت خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةoxidation_of_fats - ^ Oxidation of odd carbon chain length fatty acids
- ^ Oxidation of unsaturated fatty acids
- ^ Stryer, Lubert (1995). Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. p. 777. ISBN 0 7167 2009 4.
- ^ Sloan, A.W; Koeslag, J.H.; Bredell, G.A.G. (1973). "Body composition work capacity and work efficiency of active and inactive young men". European Journal of Applied Physiology. 32: 17–24. doi:10.1007/bf00422426.
- ^ Ruddick JA (1972). "Toxicology, metabolism, and biochemistry of 1,2-propanediol". Toxicol App Pharmacol. 21: 102–111. doi:10.1016/0041-008X(72)90032-4.
- ^ أ ب ت Glew, Robert H. "You Can Get There From Here: Acetone, Anionic Ketones and Even-Carbon Fatty Acids can Provide Substrates for Gluconeogenesis". Retrieved 7 August 2016.
- ^ أ ب ت Park, Sung M.; Klapa, Maria I.; Sinskey, Anthony J.; Stephanopoulos, Gregory (1999). "Metabolite and isotopomer balancing in the analysis of metabolic cycles: II. Applications" (PDF). Biotechnology and Bioengineering. 62 (4): 398. doi:10.1002/(sici)1097-0290(19990220)62:4<392::aid-bit2>3.0.co;2-s. ISSN 0006-3592.
- ^ Miller DN, Bazzano G; Bazzano (1965). "Propanediol metabolism and its relation to lactic acid metabolism". Ann NY Acad Sci. 119 (3): 957–973. Bibcode:1965NYASA.119..957M. doi:10.1111/j.1749-6632.1965.tb47455.x. PMID 4285478.
- ^ D. L. Vander Jagt; B. Robinson; K. K. Taylor; L. A. Hunsaker (1992). "Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic complications". The Journal of Biological Chemistry. 267 (7): 4364–4369. PMID 1537826.
- ^ أ ب Stryer, Lubert (1995). "Signal transduction cascades.". In: Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. pp. 343–350. ISBN 0 7167 2009 4.
- ^ Stryer, Lubert (1995). "Eicosanoid hormones are derived from fatty acids.". In: Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. pp. 624–627. ISBN 0 7167 2009 4.
- ^ أ ب Digestion of fats (triacylglycerols)
- ^ Hofmann AF (1963). "The function of bile salts in fat absorption. The solvent properties of dilute micellar solutions of conjugated bile salts". Biochem. J. 89: 57–68. PMC 1202272. PMID 14097367.
- ^ Stryer, Lubert (1995). "Membrane structures and dynamics.". In: Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. pp. 268–270. ISBN 0 7167 2009 4.
- ^ Dijkstra, Albert J., R. J. Hamilton, and Wolf Hamm. "Fatty Acid Biosynthesis." Trans Fatty Acids. Oxford: Blackwell Pub., 2008. 12. Print.
- ^ "MetaCyc pathway: superpathway of fatty acids biosynthesis (E. coli)".
- ^ أ ب خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةstryer - ^ Diwan, Joyce J. "Fatty Acid Synthesis." Rensselaer Polytechnic Institute (RPI) :: Architecture, Business, Engineering, IT, Humanities, Science. Web. 30 Apr. 2011. <"Archived copy". Archived from the original on 2011-06-07. Retrieved 2011-05-02.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: archived copy as title (link)>. - ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةVoet