پول-إيميل لكوك دى بوابودران
پ. إ. ف. لكوك دى بوابودران P. E. F. Lecoq de Boisbaudran | |
|---|---|
 | |
| وُلِدَ | أبريل 18, 1838 |
| توفي | مايو 28, 1912 (aged 74) باريس، فرنسا |
| المدرسة الأم | المدرسة متعددة التكنولوجيات بباريس |
| عـُرِف بـ | اكتشاف گاليوم (1875)، سماريوم (1880) وديسپروزيوم (1886) والاوروپيوم (1890) |
| الجوائز | وسام داڤي (1879) مراسل أكاديمية العلوم الفرنسية عضو أجنبي في الجمعية الملكية صليب جوقة الشرف جائزة لاكاز (10,000 فرانك) |
| السيرة العلمية | |
| المجالات | الكيمياء، المطيافية |
| أثـَّر عليه | شارل أدولف ڤورتس |
پول-إيميل (فرانسوا) لكوك دى بوابودران Paul Émile (François) Lecoq de Boisbaudran (عاش 18 أبريل 1838 – 28 مايو 1912) كان كيميائياً فرنسياً عُرف باكتشافاته للعناصر الكيميائية گاليوم، سماريوم وديسپروسيوم.[1]
حسـَّن الطرق المطيافية التي اخترعها للتو كيرشهوف. وفي 1859، قام بمسح معادن ذات خطوط طيفية غير معروفة. وبعد خمسة عشر سنة من البحث الدؤوب، اكتشف العناصر گاليوم (27 أغسطس 1875)، سماريوم (1880) وديسپروزيوم (1886) واليروپيوم (1890). كما عزل في 1885 الگادولنيوم، الذي كان قد اكتشفه گاليسار دى مارينياك (Marignac) في 1880. ويُعـَد مع بونزن وكيرشهوف وكروكس، واحداً من مؤسسي علم المطيافية. فبهدى من الترتيب العام للخطوط الطيفية للعناصر في نفس العائلة، آمن بأنه عنصر كيميائي وسماه "گاليوم". وقيل أنه اشتق الاسم من اللاتينية gallus وهي ترجمة لقب عائلته lecoq، إلا أنه نشر ورقة بحثية كذب فيها ذلك وقال أنه اشتق اسم العنصر من الاسم اللاتيني لفرنسا Gallia. وكان الگاليوم هو نفسه الإكا-ألومنيوم الذي توقعه دميتري مندليڤ بين الألومنيوم والإنديوم. ولما كان سائلاً بين درجات الحرارة 30-1700 °س، فإن الگاليوم في ثرمومتر كوارتز يمكنه قياس درجات الحرارة العالية.
أصبح مراسلاً لأكاديمية العلوم الفرنسية، وقسّم وقته بين باريس وشارنت. وفي 1894، مباشرة بعد أن اكتشف اللورد ريلي والسير وليام رامسي عنصر أرگون، افترض لكوك دى بوابودران أن العنصر عضو في مجموعة جديدة من العناصر هي الغازات النبيلة.
بعد عام 1895، مشاغل عائلية وتدهور صحته عاقا عمله. فقد عانى من تصلب المفاصل وتوفي عام 1912.
أبحاثه
Lecoq de Boisbaudran's early investigations focused on understanding the phenomenon of supersaturation, in which substances can exist in solution in higher concentrations than is possible under normal conditions. He showed that contact of supersaturated solutions with crystals of an isomorphous salt causes the substance to precipitate from the solution. He further showed that many anhydrous salts can be dissolved to create a supersaturated solution. These investigations were carried out from 1866 to 1869.[بحاجة لمصدر]
In 1874 Lecoq de Boisbaudran found that certain crystal faces dissolve more rapidly than other crystal faces. Specifically, he found that octahedral faces are less readily soluble than cubic faces in the case of ammonium alum crystals.[2]
Lecoq de Boisbaudran made major contributions to the then-new science of spectroscopy, which relates to the interaction of light and matter. He applied spectroscopy to characterize elements, particularly the rare-earth elements.[2][3][4] He developed a theoretical framework of spectroscopy, based on molecular vibrations. Theorizing that spectral frequencies relate to the atomic weight of an element, he recognized spectral trends based upon atomic masses.[5]
Boisbaudran developed new experimental apparatus and used these to carry out spectral analyses of various chemical elements.[4] Through systematic experimentation, he analysed spectra of 35 elements, using the Bunsen burner, electric spark or both to induce luminescence of samples of the elements.[2] The results of his early investigations were published in his Spectres lumineux : spectres prismatiques et en longueurs d'ondes destinés aux recherches de chimie minérale (1874).[6]
To observe spark spectra in his experimental protocols, he typically placed a solution of a salt in a sealed glass tube, with a platinum wire in the solution as a negative pole, and another platinum wire above the surface of the liquid as a positive pole.[2] In 1885, he experimented with reversing the polarity of the electric current. In this way, he obtained phosphorescent bands in the spectra providing further insight into the spectral characteristics of various chemical elements. Using this apparatus, he discovered the lanthanides samarium (1880), dysprosium (1886) and europium (1890). In 1885, he also spectroscopically characterized gadolinium in 1885, an element previously discovered in 1880 by J. C. Galissard de Marignac.[2]
سماريوم
اكتشف لكوك دى بوابودران سماريوم في 1879 بعد أن كان أول من عزل أكسيد السماريوم، تعرَّف على وجود عنصر جديد باستخدام المطيافية لملاحظة خطوط امتصاصه الضوئي الحادة المميزة.[7]
He named his new element "samaria" after the mineral samarskite from which it was isolated. The mineral itself was earlier named for a Russian mine official, Colonel Vassili Samarsky-Bykhovets. The name was eventually standardized to "samarium" to match other element names, and "samaria" now refers to the oxide of samarium, not the pure element.[8]
گاليوم
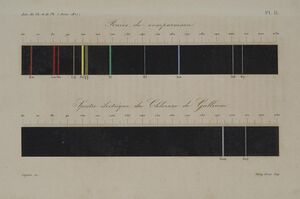
A significant achievement of Lecoq de Boisbaudran was his discovery of the element گاليوم in 1875. Beginning in 1874, Lecoq de Boisbaudran investigated a sample of 52 kg of the mineral ore sphalerite obtained from the Pierrefitte mine in the Pyrenees. From it, he extracted several milligrams of gallium chloride. Using spectroscopic methods, he observed what appeared to be two previously unreported lines in the sample's spectrum, at wavelengths of 4170 and 4031 angstroms.[2][7]
He continued his experiments using several hundred kilograms of crude zinc ore and in the same year isolated more than one gram of a near-pure metal by electrolysis of a solution of the metal in its form as a hydroxide compound, dissolved in potassium hydroxide solution. Later he prepared 75 grams of gallium using more than 4 tonnes of crude ore. He confirmed its spectral characteristics, consisting of two spectral lines in the violet portion of the spectrum of the mineral sphalerite. In this way, he ruled out the possibility that the spectral characteristics were an accident of the extraction process, rather than being an indication of a new element.[9][7]
He named his discovery "gallia", from the Latin Gallia meaning Gaul, in honor of his native land of France. It was later suggested that Lecoq de Boisbaudran had named the element after himself, since gallus is the Latin translation of the French le coq. Lecoq de Boisbaudran denied this suggestion in an article in 1877.[10] He published an account of his investigations on the new element in Sur un nouveau metal, le gallium (1877).[11]
De Boisbaudran calculated the atomic mass of gallium as 69.86, close to the currently accepted value of 69.723.[2][12] Unknown to Lecoq de Boisbaudran,[7] the existence of gallium had been predicted during 1871 by Dmitri Mendeleev, who gave it the name eka-aluminium. De Boisbaudran's discovery of gallium was significant support for Mendeleev's theory of the periodicity of the elements.[12][2][13]
ديسپروسيوم
Lecoq de Boisbaudran experimented with the precipitation of rare earth compounds from water solution using potassium sulfate to induce precipitation. He then measured the spectra of solutions in which the liquid served as a positive pole. Lecoq de Boisbaudran noted a spectral band in the yellow-green portion of the spectrum, indicative of a new element. In 1886 he succeeded in isolating a purified sample of the source of the new spectral band. He named the element dysprosium, meaning "difficult to obtain" in the Greek language.[2][14]
التصنيف الدوري للعناصر
Lecoq de Boisbaudran contributed to the development of the periodic classification of elements. This contribution occurred when he proposed that the newly discovered element argon was a member of a new, previously unrecognized, chemical series of elements that came to be known as the noble gases.[15]
الجوائز والتكريم
For his accomplishments, Lecoq de Boisbaudran was awarded the Cross of the Legion of Honour (1876),[12] the Bordin Prize from the French Academy of Sciencies (1872),[16] the Davy Medal (1879)[17][18] and the Prix Lacaze of 10,000 francs (1879).[19][20] In 1888 he was elected a foreign member of the British Royal Society.[2]
المراجع
![]() هذه المقالة تتضمن نصاً من Obituary notices، بقلم وليام رامزي، وهي مطبوعة من سنة 1913 وهي الآن مشاع عام في الولايات المتحدة.
هذه المقالة تتضمن نصاً من Obituary notices، بقلم وليام رامزي، وهي مطبوعة من سنة 1913 وهي الآن مشاع عام في الولايات المتحدة.
- ^ W. Ramsay, Joji Sakurai, K. J. P. Orton, Theodore W. Richards, W. F. Reid, Arthur R. Ling, J. T. Dunn, J. N. Collie and F. Gowland Hopkins (1913). "Obituary notices: Paul Émile (dit François) Lecoq de Boisbaudran, 1838–1912; Edward Divers, 1837–1912; Humphrey Owen Jones, F.R.S., 1878–1912; John William Mallet, 1832–1912; Henry de Mosenthal, 1850–1912; Benjamin Edward Reina Newlands, 1842–1912; John Pattinson, 1828–1912; Arthur Richardson, 1858–1912; John Wade, 1864–1912; William Ord Wootton, 1884–1912". J. Chem. Soc., Trans. 103: 742–744. doi:10.1039/CT9130300742.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ أ ب ت ث ج ح خ د ذ ر خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةRamsay - ^ DeKosky, Robert K. (1973). "Spectroscopy and the Elements in the Late Nineteenth Century: The Work of Sir William Crookes". The British Journal for the History of Science. 6 (4): 400–423. doi:10.1017/S0007087400012553. JSTOR 4025503. S2CID 146534210.
- ^ أ ب خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةMarshall - ^ Taylor, William B. (1877). "Kinetic theories of gravitation". Annual report of the Board of Regents of the Smithsonian Institution. Washington: Government Printing Office. pp. 270–271. Retrieved 4 January 2020.
- ^ Lecoq de Boisbaudran, [Paul-Émile] (1874). Spectres lumineux : spectres prismatiques et en longueurs d'ondes destinés aux recherches de chimie. Paris: Gauthier-Villars.
- ^ أ ب ت ث Weeks, Mary Elvira (1956). The discovery of the elements (6th ed.). Easton, PA: Journal of Chemical Education.
- ^ Hammond, C. R. (29 June 2004). "The Elements". Handbook of Chemistry and Physics (81st ed.). CRC press. ISBN 0-8493-0485-7.
- ^ Lecoq de Boisbaudran, Paul Émile (1875). "Caractères chimiques et spectroscopiques d'un nouveau métal, le gallium, découvert dans une blende de la mine de Pierrefitte, vallée d'Argelès (Pyrénées)". Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences. 81: 493–495.
- ^ Weeks, Mary Elvira (1932). "The discovery of the elements. XIII. Some elements predicted by Mendeleeff". Journal of Chemical Education. 9 (9): 1605–1619. Bibcode:1932JChEd...9.1605W. doi:10.1021/ed009p1605.
- ^ Lecoq de Boisbaudran, [Paul-Émile] (1877). Sur un nouveau metal, le gallium. Paris: s.n.
- ^ أ ب ت Gordin, Michael D. (11 December 2018). A well-ordered thing : Dmitrii Mendeleev and the shadow of the periodic table. Princeton University Press. pp. 36–38. ISBN 9780691172385.
- ^ Ebbing, Darrell; Gammon, Steven D. (2010). General chemistry (Enhanced., 9th ed.). Brooks/Cole Cengage Learning. p. 312. ISBN 9780538497527.
In 1874 the French chemist Paul-Émile Lecoq de Boisbaudran found two previously unidentified lines in the atomic spectrum of a sample of sphalerite (a zinc sulfide, ZnS, mineral). Realizing he was on the verge of a discovery, Lecoq de Boisbaudran quickly prepared a large batch of the zinc mineral, from which he isolated a gram of a new element. He called this new element gallium.
- ^ Emsley, John (2011). Nature's Building Blocks: An A-Z Guide to the Elements. Oxford University Press. p. 130. ISBN 978-0-19-960563-7.
- ^ Spronsen, J. W. van (1969). The periodic system of chemical elements: A history of the first hundred years. Elsevier. p. 250.
- ^ Hentschel, Klaus (2002). Mapping the spectrum : techniques of visual representation in research and teaching. Oxford University Press. pp. 118–120. ISBN 978-0198509530.
- ^ Brush, Stephen G. (2015). Making 20th century science : how theories became knowledge. Oxford University Press. pp. 161–166. ISBN 978-0-19-997815-1.
- ^ "Davy Medal". NNDB. Retrieved 28 December 2019.
- ^ "Academies et Societes Savantes". L'Année scientifique et industrielle. 24: 507. 1880. Retrieved 28 December 2019.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةGardiner
- Gardiner, J. H. (1912). "M. Lecoq De Boisbaudran". Nature. 90 (2244): 255. doi:10.1038/090255a0.