ممانتين
 | |
 | |
| البيانات السريرية | |
|---|---|
| الأسماء التجارية | Axura, Ebixa, Namenda, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604006 |
| License data | |
| فئة السلامة أثناء الحمل |
|
| مسارات الدواء | By mouth |
| رمز ATC | |
| الحالة القانونية | |
| الحالة القانونية | |
| بيانات الحركية الدوائية | |
| التوافر الحيوي | ~100% |
| الأيض | Liver (<10%) |
| Elimination half-life | 60–100 hours |
| الإخراج | Kidney |
| المعرفات | |
| |
| رقم CAS | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.217.937 |
| Chemical and physical data | |
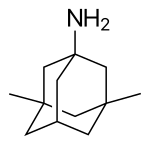
| التركيب | C12H21N |
| الكتلة المولية | 179.3 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
ممانتين، is a medication used to treat moderate-to-severe Alzheimer's disease.[2][3] It is less preferred than acetylcholinesterase inhibitors such as donepezil.[3] Treatment should only be continued if beneficial effects are seen.[3] It is taken by mouth.[2]
Common side effects include headache, constipation, sleepiness, and dizziness.[2][3] Severe side effects may include blood clots, psychosis, and heart failure.[3] It is believed to work by blocking NMDA receptors.[2]
Memantine was approved for medical use in the United States in 2003.[2] It is available as a generic medication.[3] A month supply in the United Kingdom costs the NHS about 1.60 GBP as of 2019.[3] In the United States the wholesale cost of this amount is about US$5.50.[4] In 2016 it was the 147th most prescribed medication in the United States with more than 4 million prescriptions.[5]
الاستخدام الطبي
Memantine is used to treat moderate-to-severe Alzheimer's disease, especially for people who are intolerant of or have a contraindication to AChE (acetylcholinesterase) inhibitors.[6][7] One guideline recommends memantine or an AChE inhibitor be considered in people in the early-to-mid stage of dementia.[8]
Memantine has been associated with a modest improvement;[9] with small positive effects on cognition, mood, behavior, and the ability to perform daily activities in moderate-to-severe Alzheimer's disease.[10][needs update][11] There does not appear to be any benefit in mild disease.[12]
While memantine can be used in combination with donepezil in those with dementia, the benefit of this is questionable and such dual use is not recommended by the National Institute of Clinical Excellence (NICE).[13] Memantine when added to donepezil in those with moderate to severe dementia resulted in "limited improvements" per a 2017 review.[14] Effects in autism are unclear.[15][16]
الآثار الجانبية
Memantine is, in general, well tolerated.[9] Common adverse drug reactions (≥1% of people) include confusion, dizziness, drowsiness, headache, insomnia, agitation, and/or hallucinations. Less common adverse effects include vomiting, anxiety, hypertonia, cystitis, and increased libido.[9][17]
Like many other NMDA antagonists, memantine behaves as a dissociative anesthetic at supratherapeutic doses.[18] Despite isolated reports, recreational use of memantine is rare due to the drug's long duration and limited availability.[18] Also memantine seems to lack effects such as euphoria or hallucinations.[19]
Memantine appears to be generally well tolerated by children with autism spectrum disorder.[20]
علم الأدوية
الگلوتامات
A dysfunction of glutamatergic neurotransmission, manifested as neuronal excitotoxicity, is hypothesized to be involved in the etiology of Alzheimer's disease. Targeting the glutamatergic system, specifically NMDA receptors, offers a novel approach to treatment in view of the limited efficacy of existing drugs targeting the cholinergic system.[21]
Memantine is a low-affinity voltage-dependent uncompetitive antagonist at glutamatergic NMDA receptors.[22][23] By binding to the NMDA receptor with a higher affinity than Mg2+ ions, memantine is able to inhibit the prolonged influx of Ca2+ ions, particularly from extrasynaptic receptors, which forms the basis of neuronal excitotoxicity. The low affinity, uncompetitive nature, and rapid off-rate kinetics of memantine at the level of the NMDA receptor-channel, however, preserves the function of the receptor at synapses, as it can still be activated by physiological release of glutamate following depolarization of the postsynaptic neuron.[24][25][26] The interaction of memantine with NMDA receptors plays a major role in the symptomatic improvement that the drug produces in Alzheimer's disease. However, there is no evidence as yet that the ability of memantine to protect against NMDA receptor-mediated excitotoxicity has a disease-modifying effect in Alzheimer's, although this has been suggested in animal models.[25]
السروتونين
Memantine acts as a non-competitive antagonist at the 5-HT3 receptor, with a potency similar to that for the NMDA receptor.[27] Many 5-HT3 antagonists function as antiemetics, however the clinical significance of this serotonergic activity in the treatment of Alzheimer's disease is unknown.
الكولينيات
Memantine acts as a non-competitive antagonist at different neuronal nicotinic acetylcholine receptors (nAChRs) at potencies possibly similar to the NMDA and 5-HT3 receptors, but this is difficult to ascertain with accuracy because of the rapid desensitization of nAChR responses in these experiments. It can be noted that memantine is an antagonist at Alpha-7 nAChR, which may contribute to initial worsening of cognitive function during early memantine treatment. Alpha-7 nAChR upregulates quickly in response to antagonism, which could explain the cognitive-enhancing effects of chronic memantine treatment.[28][29] It has been shown that the number of nicotinic receptors in the brain are reduced in Alzheimer's disease, even in the absence of a general decrease in the number of neurons, and nicotinic receptor agonists are viewed as interesting targets for anti-Alzheimer drugs.[30]
الدوپمين
Memantine acts as an agonist at the dopamine D2 receptor with equal or slightly higher affinity than to the NMDA receptors.[31]
مستقبلات سيگما
It acts as an agonist at the σ1 receptor with a low Ki of 2.6 μM (2600 nM).[32] The consequences of this activity are unclear (as the role of sigma receptors in general is not yet that well understood). Due to this low affinity, therapeutic concentrations of memantine are most likely too low to have any sigmaergic effect as a typical therapeutic dose is 20mg, however excessive doses of memantine taken for recreational purposes many times greater than prescribed doses may indeed activate this receptor.[33]
التاريخ
الأسماء التجارية
As of August 2017, memantine was marketed under many brand names worldwide including Abixa, Adaxor, Admed, Akatinol, Alceba, Alios, Almenta, Alois, Alzant, Alzer, Alzia, Alzinex, Alzixa, Alzmenda, Alzmex, Axura, Biomentin, Carrier, Cogito, Cognomem, Conexine, Cordure, Dantex, Demantin, Demax, Dementa, Dementexa, Ebitex, Ebixa, Emantin, Emaxin, Esmirtal, Eutebrol, Evy, Ezemantis, Fentina, Korint, Lemix, Lindex, Lindex, Lucidex, Manotin, Mantine, Mantomed, Marbodin, Mardewel, Marixino, Maruxa, Maxiram, Melanda, Memabix, Memamed, Memando, Memantin, Memantina, Memantine, Mémantine, Memantinol, Memantyn, Memanvitae, Memanxa, Memanzaks, Memary, Memax, Memexa, Memigmin, Memikare, Memogen, Memolan, Memorel, Memorix, Memotec, Memox, Memxa, Mentikline, Mentium, Mentixa, Merandex, Merital, Mexia, Mimetix, Mirvedol, Modualz, Morysa, Namenda, Nemdatine, Nemdatine, Nemedan, Neumantine, Neuro-K, Neuroplus, Noojerone, Polmatine, Prilben, Pronervon, Ravemantine, Talentum, Timantila, Tingreks, Tonibral, Tormoro, Valcoxia, Vilimen, Vivimex, Witgen, Xapimant, Xapimant, Ymana, Zalatine, Zemertinex, Zenmem, Zenmen, and Zimerz.[1]
It was also marketed in some countries as a combination drug with donepezil under the brands Namzaric, Neuroplus Dual, and Tonibral MD.[1]
الأبحاث
In ADHD evidence is not sufficient to make any conclusions.[34] It has also been studied in migraines.[35]
المصادر
- ^ أ ب ت "International brands for memantine". Drugs.com. Retrieved 7 أغسطس 2017.
- ^ أ ب ت ث ج "Memantine Hydrochloride Monograph for Professionals". Drugs.com (in الإنجليزية). American Society of Health-System Pharmacists. Retrieved 3 مارس 2019.
- ^ أ ب ت ث ج ح خ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 303–304. ISBN 9780857113382.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services (in الإنجليزية). Retrieved 3 مارس 2019.
- ^ "The Top 300 of 2019". clincalc.com. Retrieved 22 ديسمبر 2018.
- ^ Mount C, Downton C (يوليو 2006). "Alzheimer disease: progress or profit?". Nature Medicine. 12 (7): 780–4. doi:10.1038/nm0706-780. PMID 16829947.
- ^ NICE review of technology appraisal guidance 111 January 18, 2011 Alzheimer's disease - donepezil, galantamine, rivastigmine and memantine (review): final appraisal determination
- ^ Page AT, Potter K, Clifford R, McLachlan AJ, Etherton-Beer C (أكتوبر 2016). "Medication appropriateness tool for co-morbid health conditions in dementia: consensus recommendations from a multidisciplinary expert panel". Internal Medicine Journal. 46 (10): 1189–1197. doi:10.1111/imj.13215. PMC 5129475. PMID 27527376.
- ^ أ ب ت Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.
- ^ McShane R, Areosa Sastre A, Minakaran N (أبريل 2006). "Memantine for dementia". The Cochrane Database of Systematic Reviews (2): CD003154. doi:10.1002/14651858.CD003154.pub5. PMID 16625572.
- ^ van Dyck CH, Tariot PN, Meyers B, Malca Resnick E (2007). "A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease". Alzheimer Disease and Associated Disorders. 21 (2): 136–43. doi:10.1097/WAD.0b013e318065c495. PMID 17545739.
- ^ Schneider LS, Dagerman KS, Higgins JP, McShane R (أغسطس 2011). "Lack of evidence for the efficacy of memantine in mild Alzheimer disease". Archives of Neurology. 68 (8): 991–8. doi:10.1001/archneurol.2011.69. PMID 21482915.
- ^ Hersen, Michel (2006). Comprehensive handbook of personality and psychopathology (Tertiary source). Hoboken, N.J: John Wiley. p. 514. ISBN 978-0-471-75725-2. OCLC 63041762.
- ^ Chen, Ruey; Chan, Pi-Tuan; Chu, Hsin; Lin, Yu-Cih; Chang, Pi-Chen; Chen, Chien-Yu; Chou, Kuei-Ru (21 أغسطس 2017). Chen, Kewei (ed.). "Treatment effects between monotherapy of donepezil versus combination with memantine for Alzheimer disease: A meta-analysis". PLOS ONE. 12 (8): e0183586. Bibcode:2017PLoSO..1283586C. doi:10.1371/journal.pone.0183586. ISSN 1932-6203. PMC 5565113. PMID 28827830.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Parr, J (7 يناير 2010). "Autism". BMJ Clinical Evidence. 2010. PMC 2907623. PMID 21729335.
- ^ Hong, Michael P.; Erickson, Craig A. (3 أغسطس 2019). "Investigational drugs in early-stage clinical trials for autism spectrum disorder". Expert Opinion on Investigational Drugs. Informa UK Limited. 28 (8): 709–718. doi:10.1080/13543784.2019.1649656. ISSN 1354-3784. PMID 31352835.
- ^ Joint Formulary Committee (2004). British National Formulary (47th ed.). London: BMA and the Royal Pharmaceutical Society of Great Britain. ISBN 978-0-85369-584-4.
- ^ أ ب Morris H, Wallach J (2014). "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis. 6 (7–8): 614–32. doi:10.1002/dta.1620. PMID 24678061.
- ^ Swedberg MD, Ellgren M, Raboisson P (أبريل 2014). "mGluR5 antagonist-induced psychoactive properties: MTEP drug discrimination, a pharmacologically selective non-NMDA effect with apparent lack of reinforcing properties". The Journal of Pharmacology and Experimental Therapeutics. 349 (1): 155–64. doi:10.1124/jpet.113.211185. PMID 24472725.
- ^ Elbe, Dean (2019). Clinical handbook of psychotropic drugs for children and adolescents (Tertiary source). Boston, MA: Hogrefe. pp. 366–369. ISBN 978-1-61676-550-7. OCLC 1063705924.
- ^ Cacabelos R, Takeda M, Winblad B (يناير 1999). "The glutamatergic system and neurodegeneration in dementia: preventive strategies in Alzheimer's disease". International Journal of Geriatric Psychiatry. 14 (1): 3–47. doi:10.1002/(SICI)1099-1166(199901)14:1<3::AID-GPS897>3.0.CO;2-7. PMID 10029935.
- ^ Rogawski MA, Wenk GL (2003). "The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease". CNS Drug Reviews. 9 (3): 275–308. doi:10.1111/j.1527-3458.2003.tb00254.x. PMC 6741669. PMID 14530799.
- ^ Robinson DM, Keating GM (2006). "Memantine: a review of its use in Alzheimer's disease". Drugs. 66 (11): 1515–34. doi:10.2165/00003495-200666110-00015. PMID 16906789.
- ^ Xia P, Chen HS, Zhang D, Lipton SA (أغسطس 2010). "Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses". The Journal of Neuroscience. 30 (33): 11246–50. doi:10.1523/JNEUROSCI.2488-10.2010. PMC 2932667. PMID 20720132.
- ^ أ ب Parsons CG, Stöffler A, Danysz W (نوفمبر 2007). "Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse". Neuropharmacology. 53 (6): 699–723. doi:10.1016/j.neuropharm.2007.07.013. PMID 17904591.
- ^ Lipton SA (أكتوبر 2007). "Pathologically activated therapeutics for neuroprotection". Nature Reviews. Neuroscience. 8 (10): 803–8. doi:10.1038/nrn2229. PMID 17882256.
- ^ Rammes G, Rupprecht R, Ferrari U, Zieglgänsberger W, Parsons CG (يونيو 2001). "The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner". Neuroscience Letters. 306 (1–2): 81–4. doi:10.1016/S0304-3940(01)01872-9. PMID 11403963.
- ^ Buisson B, Bertrand D (مارس 1998). "Open-channel blockers at the human alpha4beta2 neuronal nicotinic acetylcholine receptor". Molecular Pharmacology. 53 (3): 555–63. doi:10.1124/mol.53.3.555. PMID 9495824.
- ^ Aracava Y, Pereira EF, Maelicke A, Albuquerque EX (مارس 2005). "Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons". The Journal of Pharmacology and Experimental Therapeutics. 312 (3): 1195–205. doi:10.1124/jpet.104.077172. PMID 15522999.
- ^ Gotti C, Clementi F (ديسمبر 2004). "Neuronal nicotinic receptors: from structure to pathology". Progress in Neurobiology. 74 (6): 363–96. doi:10.1016/j.pneurobio.2004.09.006. PMID 15649582.
- ^ Seeman P, Caruso C, Lasaga M (فبراير 2008). "Memantine agonist action at dopamine D2High receptors". Synapse. 62 (2): 149–53. doi:10.1002/syn.20472. PMID 18000814.
- ^ Peeters M, Romieu P, Maurice T, Su TP, Maloteaux JM, Hermans E (أبريل 2004). "Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine". The European Journal of Neuroscience. 19 (8): 2212–20. doi:10.1111/j.0953-816X.2004.03297.x. PMID 15090047.
- ^ "Pharms - Memantine (also Namenda) : Erowid Exp: Main Index". erowid.org. Retrieved 7 نوفمبر 2018.
- ^ Hosenbocus S, Chahal R (مايو 2013). "Memantine: a review of possible uses in child and adolescent psychiatry". Journal of the Canadian Academy of Child and Adolescent Psychiatry. 22 (2): 166–71. PMC 3647634. PMID 23667364.
- ^ Borghol A, Kirkwood A, Hawawini F (مايو 2010). "Memantine for the Treatment of Migraine". US Pharm. 35 (5): 28–35.
This NMDA receptor antagonist, approved for the treatment of Alzheimer's disease, shows promise as a prophylactic therapy for these severe headaches
قراءات إضافية
- Lipton SA (أبريل 2005). "The molecular basis of memantine action in Alzheimer's disease and other neurologic disorders: low-affinity, uncompetitive antagonism". Current Alzheimer Research. 2 (2): 155–65. doi:10.2174/1567205053585846. PMID 15974913.
وصلات خارجية
- CS1 maint: unflagged free DOI
- Short description is different from Wikidata
- Template:drugs.com link with non-standard subpage
- ECHA InfoCard ID from Wikidata
- Infobox-drug molecular-weight unexpected-character
- Pages using infobox drug with unknown parameters
- Drug has EMA link
- Wikipedia articles in need of updating from May 2019
- All Wikipedia articles in need of updating
- Use dmy dates from April 2011
- Adamantanes
- امينات
- Antidementia agents
- AbbVie Inc. brands
- Antiparkinsonian agents
- Dissociative drugs
- NMDA receptor antagonists
- ناهضات سيگما
- Treatment of Alzheimer's disease
- RTT