زبرجد زيتوني
| زبرجد زيتوني Olivine | |
|---|---|
 | |
| العامة | |
| التصنيف | Nesosilicate Olivine group Olivine series |
| الصيغة (repeating unit) | (Mg,Fe) 2SiO 4 |
| تصنيف سترونز | 9.AC.05 |
| النظام البلوري | Orthorhombic |
| التعرف | |
| Color | أصفر إلى أصفر-أخضر |
| Crystal habit | ضخم إلى حبيبي |
| Cleavage | فقير |
| Fracture | محاري - قصِف |
| Mohs scale hardness | 6.5–7 |
| Luster | مزجج |
| Streak | عديم اللون أو أبيض |
| Diaphaneity | شفاف إلى ضيائي |
| الجاذبية النوعية | 3.2–4.5[1][2][3][4] |
| الصفات البصرية | ثنائي المحاور (+) |
| Refractive index | nα = 1.630–1.650 nβ = 1.650–1.670 nγ = 1.670–1.690 |
| Birefringence | δ = 0.040 |
| References | [5][6][7] |
الزبرجد الزيتوني أو الأوليڤين (بالإنجليزية: olivine) هو معدن مركب من سيليكات المغنسيوم والحديد، وتركيبه الكيميائي (Mg,Fe)2SiO4. وهو أحد المعادن الكثيرة الوجود في القشرة الأرضية، كما يوجد في كثير من النيازك والشهب الساقطة على الأرض. كما وجد في القمر وفي المذنب 2 ويلد. تسمى الحالة النقية منه ذات البلورات الكبيرة زبرجد peridot . [9] [10]
يحتوي المعدن على خليط من السائلين الجامدين فورستريت forsterite وفياليت fayalite حيث تزيد نسبة المغنسيوم في الفورستريت وتزيد نسبة الحديد في الفياليت. ويحدد تركيب الأوليفين على أساس النسبة المولية للفورستريت (Fo) والفياليت (Fa) مثل Fo70Fa30. ويتميز الفورستريت بدرجة انصهار عالية تبلغ 1900 درجة مئوية، بينما ينصهر الفياليت عن درجة منخفضة نسبيا، عند نحو 1200 درجة مئوية. وتعتمد درجة انصهار الأوليفين على نسبة الفورستريت إلى الفياليت، كما تعتمد الخواص الأخرى للاوليفين على تلك النسبة.
التعرف عليه وتشكله

يحتوي الأوليفين عادة على كميات بسيطة من الشوائب، أهمها النيكل والمنجنيز. وهو يوجد في الصخور النارية وفي بعض الصخور المتحولة. يتبلور الأوليفين الغني بالمغنسيوم من الصهارة الغنية بالمغنسيوم والفقيرة بالسيليكا، وتكون تلك الصهارة عادة بازلت.
أما الاوليڤين الغني بالحديد فهو قليل الوجود، ويوجد في الصخور النارية وبكميات قليلة في الجرانيت و الريوليت والكوارتز. وبالعكس فلا يوجد الاوليفين الغني بالمغنسيوم في السيليكا ويكون في حالة مستقرة، إذ يتفاعل ويشكل ما يسمى اورثوپيروكسين Mg,Fe)2Si2O6).
ويـُستعمل الاوليڤين الشفاف أحياناً كحجر كريم يسمى پريدو Peridot، فرنسية تعني زيتوني. ويسمى أيضاً كريسوليت chrysolite، من الكلمات اليونانية للذهب وحجر. بعض أفضل الأحجار الكريمة من الاوليڤين تـُستخرج من كتلة صخور وشاحية في جزيرة زبرجد المصرية في البحر الأحمر.
Fe-rich olivine fayalite is relatively much less common, but it occurs in igneous rocks in small amounts in rare granites and rhyolites, and extremely Fe-rich olivine can exist stably with quartz and tridymite. In contrast, Mg-rich olivine does not occur stably with silica minerals, as it would react with them to form orthopyroxene ((Mg,Fe)
2Si
2O
6).
Mg-rich olivine is stable to pressures equivalent to a depth of about 410 km (250 mi) within Earth. Because it is thought to be the most abundant mineral in Earth's mantle at shallower depths, the properties of olivine have a dominant influence upon the rheology of that part of Earth and hence upon the solid flow that drives plate tectonics. Experiments have documented that olivine at high pressures (12 GPa, the pressure at depths of about 360 km (220 mi)) can contain at least as much as about 8900 parts per million (weight) of water, and that such water content drastically reduces the resistance of olivine to solid flow. Moreover, because olivine is so abundant, more water may be dissolved in olivine of the mantle than is contained in Earth's oceans.[11]
Olivine pine forest (a plant community) is unique to Norway. It is rare and found on dry olivine ridges in the fjord districts of Sunnmøre and Nordfjord.[12]
Olivine grains that eroded from lava on Papakolea Beach, Hawaii
Olivine in lava from the Azores
التواجد خارج كوكب الأرض
Mg-rich olivine has also been discovered in meteorites,[13] on the Moon[14] and Mars,[15][16] falling into infant stars,[17] as well as on asteroid 25143 Itokawa.[18] Such meteorites include chondrites, collections of debris from the early Solar System; and pallasites, mixes of iron-nickel and olivine. The rare A-type asteroids are suspected to have a surface dominated by olivine.[19]
The spectral signature of olivine has been seen in the dust disks around young stars. The tails of comets (which formed from the dust disk around the young Sun) often have the spectral signature of olivine, and the presence of olivine was verified in samples of a comet from the Stardust spacecraft in 2006.[20] Comet-like (magnesium-rich) olivine has also been detected in the planetesimal belt around the star Beta Pictoris.[21]
البنية البلورية
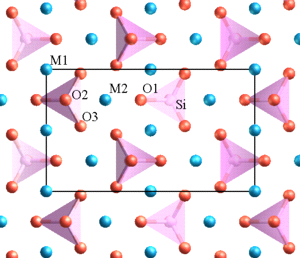
Minerals in the olivine group crystallize in the orthorhombic system (space group Pbnm) with isolated silicate tetrahedra, meaning that olivine is a nesosilicate. The structure can be described as a hexagonal, close-packed array of oxygen ions with half of the octahedral sites occupied with magnesium or iron ions and one-eighth of the tetrahedral sites occupied by silicon ions.
There are three distinct oxygen sites (marked O1, O2 and O3 in figure 1), two distinct metal sites (M1 and M2) and only one distinct silicon site. O1, O2, M2 and Si all lie on mirror planes, while M1 exists on an inversion center. O3 lies in a general position.
High-pressure polymorphs
At the high temperatures and pressures found at depth within the Earth the olivine structure is no longer stable. Below depths of about 410 km (250 mi) olivine undergoes an exothermic phase transition to the sorosilicate, wadsleyite and, at about 520 km (320 mi) depth, wadsleyite transforms exothermically into ringwoodite, which has the spinel structure. At a depth of about 660 km (410 mi), ringwoodite decomposes into silicate perovskite ((Mg,Fe)SiO
3) and ferropericlase ((Mg,Fe)O) in an endothermic reaction. These phase transitions lead to a discontinuous increase in the density of the Earth's mantle that can be observed by seismic methods. They are also thought to influence the dynamics of mantle convection in that the exothermic transitions reinforce flow across the phase boundary, whereas the endothermic reaction hampers it.[22]
The pressure at which these phase transitions occur depends on temperature and iron content.[23] At 800 °C (1،070 K; 1،470 °F), the pure magnesium end member, forsterite, transforms to wadsleyite at 11.8 جيجاباسكال (116،000 atm) and to ringwoodite at pressures above 14 GPa (138،000 atm). Increasing the iron content decreases the pressure of the phase transition and narrows the wadsleyite stability field. At about 0.8 mole fraction fayalite, olivine transforms directly to ringwoodite over the pressure range 10.0 to 11.5 GPa (99،000–113،000 atm). Fayalite transforms to Fe 2SiO 4 spinel at pressures below 5 GPa (49،000 atm). Increasing the temperature increases the pressure of these phase transitions.
التعرية
Olivine is one of the less stable common minerals on the surface according to the Goldich dissolution series. It alters into iddingsite (a combination of clay minerals, iron oxides and ferrihydrite) readily in the presence of water.[24] Artificially increasing the weathering rate of olivine, e.g. by dispersing fine-grained olivine on beaches, has been proposed as a cheap way to sequester CO2.[25][26] The presence of iddingsite on Mars would suggest that liquid water once existed there, and might enable scientists to determine when there was last liquid water on the planet.[27]
Because of its rapid weathering, olivine is rarely found in sedimentary rock.[28]
التعدين
النرويج
Norway is the main source of olivine in Europe, particularly in an area stretching from Åheim to Tafjord, and from Hornindal to Flemsøy in the Sunnmøre district. There is also olivine in Eid municipality. About 50% of the world's olivine for industrial use is produced in Norway. At Svarthammaren in Norddal olivine was mined from around 1920 to 1979, with a daily output up to 600 metric tons. Olivine was also obtained from the construction site of the hydro power stations in Tafjord. At Robbervika in Norddal municipality an open-pit mine has been in operation since 1984. The characteristic red color is reflected in several local names with "red" such as Raudbergvik (Red rock bay) or Raudnakken (Red ridge).[29][30][31][32]
Hans Strøm in 1766 described the olivine's typical red color on the surface and the blue color within. Strøm wrote that in Norddal district large quantities of olivine were broken from the bedrock and used as sharpening stones.[33]
Kallskaret near Tafjord is a nature reserve with olivine.[34]
الاستخدامات
A worldwide search is on for cheap processes to sequester CO2 by mineral reactions, called enhanced weathering. Removal by reactions with olivine is an attractive option, because it is widely available and reacts easily with the (acid) CO2 from the atmosphere. When olivine is crushed, it weathers completely within a few years, depending on the grain size. All the CO2 that is produced by burning one liter of oil can be sequestered by less than one liter of olivine. The reaction is exothermic but slow. To recover the heat produced by the reaction to produce electricity, a large volume of olivine must be thermally well-isolated. The end-products of the reaction are silicon dioxide, magnesium carbonate, and small amounts of iron oxide.[35][36] A nonprofit, Project Vesta, is investigating this approach on beaches which increase the agitation and surface area of crushed olivine through wave action.[37]
Olivine is used as a substitute for dolomite in steel works.[38]
The aluminium foundry industry uses olivine sand to cast objects in aluminium. Olivine sand requires less water than silica sands while still holding the mold together during handling and pouring of the metal. Less water means less gas (steam) to vent from the mold as metal is poured into the mold.[39]
In Finland, olivine is marketed as an ideal rock for sauna stoves because of its comparatively high density and resistance to weathering under repeated heating and cooling.[40]
Gem-quality olivine is used as a gemstone called peridot.
انظر أيضا
المصادر
| Olivine
]].- ^ Mick R. Smith (1999). Stone: Building Stone, Rock Fill and Armourstone in Construction. Geological Society of London. pp. 62–. ISBN 978-1-86239-029-4.
Specific Gravity 3.5–4.5
- ^ Jessica Elzea Kogel (2006). Industrial Minerals & Rocks: Commodities, Markets, and Uses. SME. pp. 679–. ISBN 978-0-87335-233-8.
The specific gravity is approximately 3.2 when pure rises with increasing iron content.
- ^ "Olivine". Science.smith.edu. Archived from the original on 2014-01-20. Retrieved 2013-11-14.
G = 3.22 to 4.39. Specific gravity increases and hardness decreases with increasing Fe.
- ^ "University of Minnesota's Mineral Pages: Olivine". Geo.umn.edu. Archived from the original on 2013-10-17. Retrieved 2013-11-14.
Specific Gravity: 3.2 (Mg-rich variety) to 4.3 (Iron-rich variety) (average weight)
- ^ Olivine Archived 2014-12-09 at the Wayback Machine. Webmineral.com Retrieved on 2012-06-16.
- ^ Olivine Archived 2008-02-02 at the Wayback Machine. Mindat.org Retrieved on 2012-06-16.
- ^ Klein, Cornelis; C. S. Hurlburt (1985). Manual of Mineralogy (20th ed.). New York: John Wiley & Sons. ISBN 978-0-471-80580-9.
- ^ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- ^ Fukang and other Pallasites
- ^ Pretty Green Mineral....
- ^ Smyth, J. R.; Frost, D. J.; Nestola, F.; Holl, C. M.; Bromiley, G. (2006). "Olivine hydration in the deep upper mantle: Effects of temperature and silica activity" (PDF). Geophysical Research Letters. 33 (15): L15301. Bibcode:2006GeoRL..3315301S. CiteSeerX 10.1.1.573.4309. doi:10.1029/2006GL026194. S2CID 35342757. Archived from the original (PDF) on 2017-08-09. Retrieved 2017-10-26.
- ^ Brandrud, T.E. (2009). "Olivinfuruskog og rødlistearter i Bjørkedalen, Volda: naturverdi og forvaltningsmuligheter". NINA Rapport (in norwegian). 461. Retrieved 14 February 2021.
{{cite journal}}: CS1 maint: unrecognized language (link) - ^ Fukang and other Pallasites Archived 2008-12-21 at the Wayback Machine. Farlang.com (2008-04-30). Retrieved on 2012-06-16.
- ^ Meyer, C. (2003). "Mare Basalt Volcanism" (PDF). NASA Lunar Petrographic Educational Thin Section Set. NASA. Archived (PDF) from the original on 21 December 2016. Retrieved 23 October 2016.
- ^ Pretty Green Mineral.... Archived 2007-05-04 at the Wayback MachineMission Update 2006... Archived 2010-06-05 at the Wayback Machine UMD Deep Impact Website, University of Maryland Ball Aerospace & Technology Corp. retrieved June 1, 2010
- ^ Hoefen, T.M., et al. 2003. "Discovery of Olivine in the Nili Fossae Region of Mars". Science 302, 627–30. "Hoefen, T. M. (2003). "Discovery of Olivine in the Nili Fossae Region of Mars". Science. 302 (5645): 627–630. Bibcode:2003Sci...302..627H. doi:10.1126/science.1089647. PMID 14576430. S2CID 20122017."
- ^ Spitzer Sees Crystal Rain... Archived 2011-05-29 at the Wayback Machine NASA Website
- ^ Japan says Hayabusa brought back asteroid grains... Archived 2010-11-18 at the Wayback Machine retrieved November 18, 2010
- ^ Sanchez, Juan A.; et al. (January 2014). "Olivine-dominated asteroids: Mineralogy and origin". Icarus. 228: 288–300. arXiv:1310.1080. Bibcode:2014Icar..228..288S. doi:10.1016/j.icarus.2013.10.006. S2CID 42791787.
- ^ Press Release 06-091 Archived 2006-08-28 at the Wayback Machine. Jet Propulsion Laboratory Stardust website, retrieved May 30, 2006.
- ^ De Vries, B. L.; Acke, B.; Blommaert, J. A. D. L.; Waelkens, C.; Waters, L. B. F. M.; Vandenbussche, B.; Min, M.; Olofsson, G.; Dominik, C.; Decin, L.; Barlow, M. J.; Brandeker, A.; Di Francesco, J.; Glauser, A. M.; Greaves, J.; Harvey, P. M.; Holland, W. S.; Ivison, R. J.; Liseau, R.; Pantin, E. E.; Pilbratt, G. L.; Royer, P.; Sibthorpe, B. (2012). "Comet-like mineralogy of olivine crystals in an extrasolar proto-Kuiper belt". Nature. 490 (7418): 74–76. arXiv:1211.2626. Bibcode:2012Natur.490...74D. doi:10.1038/nature11469. PMID 23038467. S2CID 205230613.
- ^ Christensen, U.R. (1995). "Effects of phase transitions on mantle convection". Annu. Rev. Earth Planet. Sci. 23: 65–87. Bibcode:1995AREPS..23...65C. doi:10.1146/annurev.ea.23.050195.000433.
- ^ Deer, W. A.; R. A. Howie; J. Zussman (1992). An Introduction to the Rock-Forming Minerals (2nd ed.). London: Longman. ISBN 978-0-582-30094-1.
- ^ Kuebler, K.; Wang, A.; Haskin, L. A.; Jolliff, B. L. (2003). "A Study of Olivine Alteration to Iddingsite Using Raman Spectroscopy" (PDF). Lunar and Planetary Science. 34: 1953. Bibcode:2003LPI....34.1953K. Archived (PDF) from the original on 2012-10-25.
- ^ Goldberg, Philip; Chen Zhong-Yin; Connor, William'O; Walters, Richards; Ziock, Hans (2001). "CO2 Mineral Sequestration Studies in US" (PDF). Archived from the original (PDF) on 2016-12-21. Retrieved 2016-12-19.
- ^ Schuiling, R.D.; Tickell, O. "Olivine against climate change and ocean acidification" (PDF). Archived from the original (PDF) on 2016-09-27. Retrieved 2016-12-19.
- ^ Swindle, T. D.; Treiman, A. H.; Lindstrom, D. J.; Burkland, M. K.; Cohen, B. A.; Grier, J. A.; Li, B.; Olson, E. K. (2000). "Noble Gases in Iddingsite from the Lafayette meteorite: Evidence for Liquid water on Mars in the last few hundred million years". Meteoritics and Planetary Science. 35 (1): 107–15. Bibcode:2000M&PS...35..107S. doi:10.1111/j.1945-5100.2000.tb01978.x.
- ^ Velbel, Michael A. (October 2009). "Dissolution of olivine during natural weathering". Geochimica et Cosmochimica Acta. 73 (20): 6098–6113. Bibcode:2009GeCoA..73.6098V. doi:10.1016/j.gca.2009.07.024.
- ^ Furseth, Astor (1987): Norddal i 150 år. Valldal: Norddal kommune.
- ^ Geological Survey of Norway. Kart over mineralressurser Archived 2017-10-14 at the Wayback Machine. Accessed 9.12.2012.
- ^ "Olivin". www.ngu.no (in النرويجية بوكمال). Archived from the original on 2017-11-10. Retrieved 2017-11-09.
- ^ Gjelsvik, T. (1951). Oversikt over bergartene i Sunnmøre og tilgrensende deler av Nordfjord Archived 2017-11-10 at the Wayback Machine. Norge geologiske undersøkelser, report 179.
- ^ Strøm, Hans: Physisk og Oeconomisk Beskrivelse over Fogderiet Søndmør beliggende i Bergen Stift i Norge. Published in Sorø, Denmark, 1766.
- ^ "Kallskaret". 28 September 2014. Archived from the original on 10 November 2017. Retrieved 3 May 2018 – via Store norske leksikon.
- ^ Goldberg, P.; Chen, Z.-Y.; O'Connor, W.; Walters, R.; Ziock, H. (2000). "CO2 Mineral Sequestration Studies in US" (PDF). Technology. 1 (1): 1–10. Archived from the original (PDF) on 2003-12-07. Retrieved 2008-07-07.
- ^ Schuiling, R. D.; Krijgsman, P. (2006). "Enhanced Weathering: An Effective and Cheap Tool to Sequester CO2". Climatic Change. 74 (1–3): 349–54. Bibcode:2006ClCh...74..349S. doi:10.1007/s10584-005-3485-y. S2CID 131280491.
- ^ Delbert, Caroline (2020-06-11). "How This Strange Green Sand Could Reverse Climate Change". Popular Mechanics (in الإنجليزية الأمريكية). Retrieved 2020-11-06.
- ^ Mineralressurser i Norge ; Mineralstatistikk og bergverksberetning 2006. Trondheim: Bergvesenet med bergmesteren for Svalbard. 2007.
- ^ Ammen, C. W. (1980). The Metal Caster's Bible. Blue Ridge Summit PA: TAB. p. 331. ISBN 978-0-8306-9970-4.
- ^ "The olivine stone". Suomen Kiuaskivi. Retrieved 14 February 2021.