خلات الفضة
| |
| |
| الأسماء | |
|---|---|
| اسم أيوپاك
Silver(I) acetate
| |
| اسم أيوپاك النظامي
Silver(I) ethanoate | |
| أسماء أخرى
Acetic acid, silver(I) salt
Silver ethanoate Argentous acetate Argentous ethanoate | |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.414 |
| رقم EC |
|
PubChem CID
|
|
| رقم RTECS |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | AgC2H3O2 |
| كتلة مولية | 166.912 g/mol |
| المظهر | white to slightly grayish powder slightly acidic odor |
| الكثافة | 3.26 g/cm3, solid |
| نقطة الغليان | |
| قابلية الذوبان في الماء | 1.02 g/100 mL(20 °C) |
| نتاج قابلية الذوبان، Ksp | 1.94×10−3[1] |
| القابلية المغناطيسية | −60.4·10−6 cm3/mol |
| المخاطر | |
| ن.م.ع. مخطط تصويري |  
|
| ن.م.ع. كلمة الاشارة | Warning |
| H315, H319, H335, H400 | |
| P261, P264, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P391, P403+P233, P405, P501 | |
| NFPA 704 (معيـَّن النار) | |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
خلات الفضة مركب كيميائي له الصيغة AgC2H3O2 ، ويكون على شكل مسحوق بلوري أبيض إلى رمادي.
التحضير
Silver acetate can be synthesized by the reaction of acetic acid and silver carbonate.[3]
- 2 CH3CO2H + Ag2CO3 → 2 AgO2CCH3 + H2O + CO2
Solid silver acetate precipitates upon concentration of solutions of silver nitrate and sodium acetate.
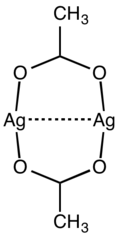
The structure of silver acetate consists of 8-membered Ag2O4C2 rings formed by a pair of acetate ligands bridging a pair of silver centres.[4]
التفاعلات
Silver acetate finds use in certain transformations in organic synthesis.[5]
Sulfenamide synthesis
Silver acetate is used to prepare sulfenamides from disulfides and secondary amines:[5]
- R2NH + AgOAc + (RS)2 → R2NSR + AgSR + HOAc
الهدرجة
A solution of silver acetate in pyridine absorbs hydrogen, producing metallic silver:[6]
- 2 CH3CO2Ag + H2 → 2 Ag + 2 CH3CO2H
Direct ortho-arylation
Silver acetate is a reagent for direct ortho-arylation (to install two adjacent substituents on an aromatic ring) of benzylamines and N-methylbenzylamines. The reaction is palladium-catalyzed and requires a slight excess of silver acetate.[7] This reaction is shorter than previous ortho-arylation methods.
Oxidative dehalogenation
Silver acetate can be used to convert certain organohalogen compounds into alcohols. It may be used, in spite of its high cost, in instances where a mild and selective reagent is desired.
Woodward cis-hydroxylation
Silver acetate in combination with iodine forms the basis of the Woodward cis-hydroxylation. This reaction selectively converts an alkene into a cis-diol.[8]
الاستخدامات
In the health field, silver acetate-containing products have been used in gum, spray, and lozenges to deter smokers from smoking. The silver in these products, when mixed with smoke, creates an unpleasant metallic taste, thus deterring them from smoking. Lozenges containing 2.5 mg of silver acetate showed "modest efficacy" on 500 adult smokers tested over a three-month period. However, over a period of 12 months, prevention failed. In 1974, silver acetate was first introduced in Europe as an over-the-counter smoking-deterrent lozenge (Repaton) and then three years later as a chewing gum (Tabmint).[9]
Silver acetate is also a well known precursor used in printed electronics. Particularly, complexes of silver acetate have been reported to form particle free "reactive inks" that form traces that approach bulk silver conductivity (within one order of magnitude).[10]
السلامة
The LD50 of silver acetate in mice is 36.7 mg/kg. Low doses of silver acetate in mice produced hyper-excitability, ataxia, central nervous system depression, labored breathing, and even death.[11] The U.S. FDA recommends that silver acetate intake be limited to 756 mg over a short period of time; excessive intake may cause argyria.[9][12]
المراجع
- ^ John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (in English) (99 ed.). CRC Press. pp. 5–189. ISBN 978-1138561632.
{{cite book}}: CS1 maint: unrecognized language (link) - ^ "Silver acetate". pubchem.ncbi.nlm.nih.gov (in الإنجليزية). Retrieved 15 December 2021.
- ^ Logvinenko, V.; Polunina, O.; Mikhailov, Yu; Mikhailov, K.; Bokhonov, B. (2007). "Study of Thermal Decomposition of Silver Acetate". Journal of Thermal Analysis and Calorimetry. 90 (3): 813–816. doi:10.1007/s10973-006-7883-9. S2CID 96769867.
- ^ Olson, Leif P.; Whitcomb, David R.; Rajeswaran, Manju; Blanton, Thomas N.; Stwertka, Barbara J. (2006). "The Simple Yet Elusive Crystal Structure of Silver Acetate and the Role of the Ag−Ag Bond in the Formation of Silver Nanoparticles during the Thermally Induced Reduction of Silver Carboxylates". Chemistry of Materials. 18 (6): 1667–1674. doi:10.1021/cm052657v.
- ^ أ ب "Silver(I) Acetate". Encyclopedia of Reagents for Organic Synthesis. 2008. doi:10.1002/047084289X.rs013m.pub2. ISBN 978-0471936237.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Wright, Leon; Well, Sol; Mills, G.A. (1955). "Homogeneous Catalytic Hydrogenation III. Activation of Hydrogen by Cuprous and Silver Acetates in Pyridine and Dodecylamine". Journal of Physical Chemistry. 59 (10): 1060–1064. doi:10.1021/j150532a016.
- ^ Lazareva, Anna; Daugulis, Olafs (2006). "Direct Palladium-Catalyzed Ortho-Arylation of Benzylamines". Organic Letters. 8 (23): 5211–5213. doi:10.1021/ol061919b. PMID 17078680.
- ^ Woodward, R. B.; Brutcher, F. V. (January 1958). "cis-Hydroxylation of a Synthetic Steroid Intermediate with Iodine, Silver Acetate and Wet Acetic Acid". Journal of the American Chemical Society. 80 (1): 209–211. doi:10.1021/ja01534a053.
- ^ أ ب Hymowitz, Norman; Eckholdt, Haftan (1996). "Effects of a 2.5-mg Silver Acetate Lozenge on Initial and Long-Term Smoking Cessation". Journal of Preventive Medicine. 25 (5): 537–546. doi:10.1006/pmed.1996.0087. PMID 8888321.
- ^ "Reactive Silver Inks for High-Performance Printed Electronics". Sigma-Aldrich (in الإنجليزية). Retrieved 2019-08-11.
- ^ Horner, Heidi C.; Roebuck, B.D.; Smith, Roger P.; English, Jackson P. (1977). "Acute toxicity of some silver salts of sulfonamides in mice and the efficacy of penicillamine in silver poisoning". Drug and Chemical Toxicology. 6 (3): 267–277. doi:10.3109/01480548309017817. PMID 6628259.
- ^ E. J. Jensen; E. Schmidt; B. Pedersen; R. Dahl (1991). "Effect on smoking cessation of silver acetate, nicotine and ordinary chewing gum, Influence of smoking history". Psychopharmacology. 104 (4): 470–474. doi:10.1007/BF02245651. PMID 1780416. S2CID 1411297.
المصادر
- F. H. MacDougall; S. Peterson (1947). "Equilibria in Silver Acetate Solutions". The Journal of Physical Chemistry. 51 (6): 1346–1361. doi:10.1021/j150456a009. PMID 20269041.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help)
| أملاح وإستر أيون الخلات | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | ROAc | NH4OAc | AcOAc | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 | Mn(OAc)2 MnAc3 |
Fe(OAc)2 FeAc3 |
Co(OAc)2, CoAc3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru | Rh | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 SnAc4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, HgAc2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
