جهاز المساعدة الأذينية
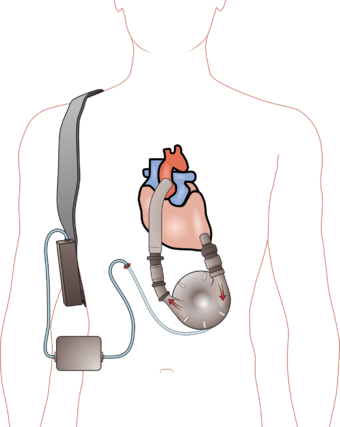
Graphic describing a Left Ventricular Assist Device (LVAD). The device pumps blood from the left ventricle to the aorta.
جهاز المساعدة الأذينية Ventricular assist device، أو VAD، هو جهاز يستخدم ليقوم بوظيفة القلب بصفة جزئية أو كاملة في حالات فشل القلب. بعض أجهزة المساعدة الأذينية تستخدم لفترات قصيرة، مثل تلك التي تستخدم لعلاج النوبات القلبية أو جراحة القلب، بينما توجد أجهزة أخرى تستخدم لفترات طويلة (شهور أو سنوات وفي بعض الأحيان طول العمر)، مثل التي يستخدمها المرضى المصابون بفشل القلب الاحتقاني.
التصميم
المضخات
التاريخ
الدراست والأبحاث
المضاعفات والآثار الجانبية
قائمة أجهزة المساعدة الأذينية
هذه قائمة بأهزة المساعدة الأذينية
| الجهاز | المصنع | النوع | الموافقة على استخدامه في يوليو 2009 |
|---|---|---|---|
| Novacor | World Heart | Pulsatile. | Was approved for use in North America, European Union and Japan. Now defunct and no longer supported by the manufacturer. |
| HeartMate XVE | Thoratec | Pulsatile. | FDA approval for BTT in 2001 and DT in 2003. CE Mark Authorized. Rarely used anymore due to reliability concerns. |
| HeartMate II | Thoratec | Rotor driven continuous axial flow, ball and cup bearings. | Approved for use in North America and EU. CE Mark Authorized. FDA approval for BTT in April 2008. Recently approved by FDA in the US for Destination Therapy (as at January 2010). |
| HeartMate III | Thoratec | Continuous flow driven by a magnetically suspended axial flow rotor. | Clinical trials yet to start, uncertain future. |
| Incor | Berlin Heart | Continuous flow driven by a magnetically suspended axial flow rotor. | Approved for use in European Union. Used on humanitarian approvals on case by case basis in the US. Entered clinical trials in the US in 2009. |
| Jarvik 2000 | Jarvik Heart | Continuous flow, axial rotor supported by ceramic bearings. | Currently used in the United States as a bridge to heart transplant under an FDA-approved clinical investigation. In Europe, the Jarvik 2000 has earned CE Mark certification for both bridge-to-transplant and lifetime use. Child version currently being developed. |
| MicroMed DeBakey VAD | MicroMed | Continuous flow driven by axial rotor supported by ceramic bearings. | Approved for use in the European Union. The child version is approved by the FDA for use in children in USA. Undergoing clinical trials in USA for FDA approval. |
| VentrAssist[dead link] | Ventracor[1] | Continuous flow driven by a hydrodynamically suspended centrifugal rotor. | Approved for use in European Union and Australia. Company declared bankrupt while clinical trials for FDA approval were underway in 2009. Company now dissolved and intellectual property sold to Thoratec. |
| MTIHeartLVAD | MiTiHeart Corporation | Continuous flow driven by a magnetically suspended centrifugal rotor. | Yet to start clinical trials. |
| C-Pulse | Sunshine Heart | Pulsatile, driven by an inflatable cuff around the aorta. | Currently in clinical trials in the US and Australia. |
| HVAD | HeartWare | Miniature "third generation" device with centrifugal blood path and hydromagnetically suspended rotor that may be placed in the pericardial space. | Obtained CE Mark for distribution in Europe, January 2009. Initiated US BTT trial in October 2008 (completed February 2010) and US DT trial in August 2010. |
| DuraHeart | Terumo | Magnetically levitated centrifugal pump. | CE approved, US FDA trials underway as at January 2010. |
| Thoratec PVAD (Paracorporeal Ventricular Assist Device) | Thoratec | Pulsatile system includes three major components: Blood pump, cannulae and pneumatic driver (dual drive console or portable VAD driver). | CE Mark Authorized. Received FDA approval for BTT in 1995 and for post-cardiotomy recovery (open heart surgery) in 1998. |
| IVAD - Implantable Ventricular Assist Device | Thoratec | Pulsatile system includes three major components: Blood pump, cannulae and pneumatic driver (dual drive console or portable VAD driver). | CE Mark Authorized. Received FDA approval for BTT in 2004. Authorized only for internal implant, not for paracorporeal implant due to reliability issues. |
انظر أيضا
المصادر
- ^ Ventracor was put into liquidation on on July 3, 2009, whereby the company's assets including its intellectual property, data from clinical trials, plant and equipment and residual assets will be put up for saleBoyd, Tony (2009-07-13). "No Heart". Business Spectator. Retrieved 15 September 2009.
{{cite news}}: Cite has empty unknown parameters:|trans_title=and|coauthors=(help)
وصلات خارجية
- LifeFlow LVAD at the University of Virginia
- Nader Moazami, Patrick M. McCarthy Temporary Circulatory Support
- Eugene L. Kukuy, Mehmet C. Oz, Yoshifumi Naka Long-Term Mechanical Circulatory Support - a review of the subject as at 2003.
- Mechanical Circulatory Support Resource Center
- Health Center Online VAD
- Mayo Clinic VAD
- Life without a pulse — news story about Canadian man with VAD
- Heart Pump Design Could Give Patients New Hope — A new counter-flow heart pump developed by Queensland University of Technology
- Heart pump improves quality of life in congestive heart failure patients A rapid review of the medical literature and specialist opinion as at December 2005
- NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE (UK) Interventional procedures overview - short-term circulatory support with left ventricular assist devices as a bridge to cardiac transplantation or recovery A rapid review of the medical literature and specialist opinion as at December 2005
- Courtney J. Gemmato,Matthew D. Forrester,Timothy J. Myers,O.H. Frazier,Denton A. Cooley Thirty-Five Years of Mechanical Circulatory Support at the Texas Heart Institute Tex Heart Inst J 2005;32:168-77
This article may include material from Wikimedia licensed under CC BY-SA 4.0. Please comply with the license terms.