إنسولين أسپارت
 | |
| البيانات السريرية | |
|---|---|
| الأسماء التجارية | NovoLog, NovoRapid, Fiasp, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605013 |
| License data | |
| مسارات الدواء | Subcutaneous, intravenous |
| رمز ATC | |
| الحالة القانونية | |
| الحالة القانونية | |
| المعرفات | |
| رقم CAS | |
| PubChem CID | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| التركيب | C256H381N65O79S6 |
| الكتلة المولية | 5825.8 g/mol |
| | |
إنسولين أسپارت، يُباع تحت الاسم التجاري نوڤولوگ Novolog، هو نوع من الإنسولين المُصنع الذي يُستخدم لعلاج سكري النمط الأول والثاني.[1] Typically it is taken just before eating.[1] It is generally used by injection under the skin but may also be used by injection into a vein.[1] Maximum effect occurs after about 1-3 hours and lasts for 3-5 hours.[1] Generally a longer-acting insulin like NPH is also needed.[1]
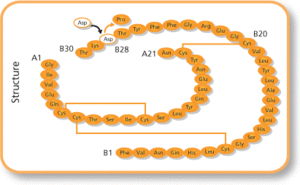
Common side effects include low blood sugar, allergic reactions, itchiness, and pain at the site of injection.[1] Other serious side effects may include low blood potassium.[1] Use in pregnancy and breastfeeding is generally safe.[2] It works the same as human insulin by increasing the amount of glucose that tissues take in and decreasing the amount of glucose made by the liver.[1] It is a manufactured form of human insulin; where a single amino acid has been changed, specifically a proline with an aspartic acid at the B28 position.[3]
Insulin aspart was approved for medical use in the United States in 2000.[1] In the United Kingdom it costs the NHS about £1.89 per 100 units as of 2019.[4] In the United States the wholesale cost of this amount is about US$30.00.[5] In 2016 it was the 87th most prescribed medication in the United States with more than 8 million prescriptions.[6] Manufacturing involves yeast, which have had the gene for insulin aspart put into their genome.[7] This yeast then makes the insulin, which is harvested from the bioreactor.[8]
الاستخدامات الطبية
As of 2018 there is a lack of compelling evidence to conclude superiority of insulin aspart over human insulin in type 2 DM.[9] It is thus unclear why the shifting of people from human insulin to insulin aspart has occurred.[10] In type 1 DM it appears to result in slightly better blood sugar control.[11]
بداية التأثير
The onset of action is approximately 15 minutes, the peak action is reached in 45–90 minutes, and the duration is 3–5 hours. However, as with all insulin, these numbers are based on averages, and vary between individuals due to blood flow, injection site, temperature and exercise.[12][13]
الآثار الجانبية
The safety of insulin aspart in people with diabetes is no different than for regular insulin. The side effects that are commonly associated with insulin therapy include: allergic reactions, injection site irritation, rashes, and hypoglycemia.[14] The most common side effect is hypoglycemia. Long-term use of insulin, including insulin aspart, can cause lipodystrophy at the site of repeated injections or infusion. To reduce the risk of lipodystrophy, rotate the injection sites within the same region. Weight gain can also occur with the use of insulin aspart and it has been attributed to anabolic effects of insulin and a decrease in glucosuria. Use of insulin aspart has also been associated with sodium retention and edema.[15]
الخصائص الكيميائية
The components of insulin aspart are as follows:
- Metal ion – zinc (19.6 μg/mL)
- Buffer – disodium hydrogen phosphate dihydrate (1.25 mg/mL)
- Preservatives – 3-methylphenol (m-cresol) (1.72 mg/mL) and phenol (1.50 mg/mL)
- Isotonicity agents – glycerin (16 mg/mL) and sodium chloride (0.58 mg/mL).
The pH of insulin aspart is 7.2–7.6.[12]
التركيبات
Insulin aspart can be used in an insulin pump and insulin pen for subcutaneous injection. Additionally, it can be used with an injection port such as the I-port.[16]
Insulin aspart has a more rapid onset, and a shorter duration of activity than normal human insulin. Therefore, insulin aspart given by injection should normally be used in a regimen with long-acting or intermediate insulin.[17] Insulin aspart can also be used with external insulin pumps. The insulin in reservoirs of insulin pumps and infusion sets should be changed every 48 hours to avoid insulin degradation and loss of preservative.[13]
درجة الحرارة
It has been debated whether or not insulin aspart should be kept refrigerated at all times. The manufacturer claims it can last 28 days without refrigeration, as long as it is kept below 86 °F / 30 °C.[18] Above these temperatures, the potency of the insulin quickly degrades, rendering it less effective.
الأنواع
NovoLog Mix 70/30 is a product which contains 30% insulin aspart and 70% insulin aspart protamine. The insulin aspart protamine portion is a crystalline form of insulin aspart, which delays the action of the insulin, giving it a prolonged absorption profile after injection. The combination of the fast-acting form and the long-acting form allows the patient to receive fewer injections over the course of the day.[19]
The components of NovoLog Mix 70/30 are as follows:
- Metal ion – zinc (19.6 μg/mL)
- Buffer – dibasic sodium phosphate (1.25 mg/mL)
- Preservatives – m-cresol (1.72 mg/mL) and phenol (1.50 mg/mL)
- Isotonicity agents – sodium chloride (0.58 mg/mL) and mannitol (36.4 mg/mL)
- Modifying protein – protamine (0.33 mg/mL)
The pH is 7.2–7.44.[12]
NovoLog Mix is marketed to be used with the Novo Nordisk FlexPen.[20] The onset of action is less than 30 minutes, the peak action is reached in 1–4 hours, and the duration is less than 24 hours.[12] NovoLog Mix is marketed in some countries as NovoMix 30.[21]
المصادر
- ^ أ ب ت ث ج ح خ د ذ "Insulin Aspart Monograph for Professionals". Drugs.com (in الإنجليزية). American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- ^ "Insulin aspart Pregnancy and Breastfeeding Warnings". Drugs.com (in الإنجليزية). Retrieved 3 March 2019.
- ^ Turner JR (2010). New Drug Development: An Introduction to Clinical Trials: Second Edition (in الإنجليزية). Springer Science & Business Media. p. 32. ISBN 9781441964182.
- ^ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 697. ISBN 9780857113382.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services (in الإنجليزية). Retrieved 3 March 2019.
- ^ "The Top 300 of 2019". clincalc.com. Retrieved 22 December 2018.
- ^ Banga, Ajay K. (2005). Therapeutic Peptides and Proteins: Formulation, Processing, and Delivery Systems, Second Edition (in الإنجليزية). CRC Press. p. 13. ISBN 9781420039832.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Schmid, Rolf D.; Schmidt-Dannert, Claudia (2016). Biotechnology: An Illustrated Primer (in الإنجليزية). John Wiley & Sons. p. 222. ISBN 9783527677566.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Fullerton B, Siebenhofer A, Jeitler K, Horvath K, Semlitsch T, Berghold A, Gerlach FM (December 2018). "Short-acting insulin analogues versus regular human insulin for adult, non-pregnant persons with type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews. 12: CD013228. doi:10.1002/14651858.CD013228. PMC 6517032. PMID 30556900.
- ^ Cohen D, Carter P (December 2010). "How small changes led to big profits for insulin manufacturers". BMJ. 341: c7139. doi:10.1136/bmj.c7139. PMID 21159773.
- ^ Fullerton B, Siebenhofer A, Jeitler K, Horvath K, Semlitsch T, Berghold A, et al. (June 2016). "Short-acting insulin analogues versus regular human insulin for adults with type 1 diabetes mellitus". The Cochrane Database of Systematic Reviews (6): CD012161. doi:10.1002/14651858.CD012161. PMC 6597145. PMID 27362975.
- ^ أ ب ت ث Crommelin DJA, Sindelar RD, Meibohm B. 2008. Pharmaceutical Biotechnology: Fundamentals and Applications. New York, NY: Informa Healthcare USA, Inc. p 270.
- ^ أ ب "NovoLog Insulin Aspart (rDNA origin) Injection" (PDF). FDA. 7 June 2000.
- ^ "Novolog: most FAQ". Archived from the original on 2011-10-03. Retrieved 2011-04-18.
- ^ "Novolog: insulin aspart (rDNA origin) injection" (PDF). Novo Nordisk.
- ^ "Aspart insulin (rDNA origin) injection". Archived from the original on 2007-06-10. Retrieved 2007-06-08.
- ^ "Novolog: insulin aspart" (PDF).
- ^ "insulin aspart Storage". Novolog.com. Retrieved 2016-04-23.
- ^ Rx List: NovoLog Mix 70/30. 6 August 2008. http://www.rxlist.com/novolog-mix-70-30-drug.htm
- ^ Novo Nordisk: NovoLog Mix 70/30. 2008. http://www.novologmix70-30.com/starting-with-novolog-mix.asp Archived 2009-05-08 at the Wayback Machine
- ^ "NovoMix 30 Penfill 100 units/ml, NovoMix 30 FlexPen 100 units/ml - Summary of Product Characteristics (SmPC) - (eMC)". www.medicines.org.uk.
- CS1 errors: unsupported parameter
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- Chemicals that do not have a ChemSpider ID assigned
- Articles with changed EBI identifier
- Infobox-drug molecular-weight unexpected-character
- Pages using infobox drug with unknown parameters
- Infobox drug articles with non-default infobox title
- Articles without InChI source
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- RTT
- Insulin receptor agonists
- علاج الإنسولين
- السكري
- پروتينات بشرية
- پروتينات مؤشبة
- هرمونات پپتيدية