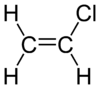
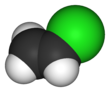
كلوريد الڤاينيل
|
| |||
| الأسماء | |||
|---|---|---|---|
| اسم أيوپاك
Chloroethene
| |||
| أسماء أخرى
مونومر كلوليد الڤاينيل
VCM Chloroethylene Refrigerant-1140 | |||
| المُعرِّفات | |||
| رقم CAS | |||
3D model (JSmol)
|
|||
| مرجع بايلستاين | 1731576 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.756 | ||
| رقم EC |
| ||
| مرجع Gmelin | 100541 | ||
| KEGG | |||
PubChem CID
|
|||
| رقم RTECS |
| ||
| UNII | |||
| UN number | 1086 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| الخصائص | |||
| الصيغة الجزيئية | C2H3Cl | ||
| كتلة مولية | 62.5 g mol-1 | ||
| المظهر | Colorless gas | ||
| الرائحة | pleasant[1] | ||
| الكثافة | 0.911 g/cc | ||
| نقطة الانصهار | |||
| نقطة الغليان | |||
| قابلية الذوبان في الماء | 2.7 g/L (0.0432 mol/L) | ||
| ضغط البخار | 2580 mmHg at 20 °C (68 °F) | ||
| القابلية المغناطيسية | -35.9·10−6 cm3/mol | ||
| الكيمياء الحرارية | |||
| الإنتالپية المعيارية للتشكل ΔfH |
−94.12 kJ/mol (solid) | ||
| سعة الحرارة النوعية، C | 0.8592 J/K/g (gas) 0.9504 J/K/g (solid) | ||
| المخاطر | |||
| ن.م.ع. مخطط تصويري |  
| ||
| ن.م.ع. كلمة الاشارة | Danger | ||
| H220, H350 | |||
| P201, P202, P210, P281, P308+P313, P377, P381, P403, P405, P501 | |||
| NFPA 704 (معيـَّن النار) | |||
| نقطة الوميض | −61 °C (−78 °F; 212 K) | ||
| حدود الانفجار | 3.6–33%[1] | ||
| حدود التعرض الصحية بالولايات المتحدة (NIOSH): | |||
PEL (المسموح)
|
TWA 1 ppm C 5 ppm [15-minute][1] | ||
REL (الموصى به)
|
Ca[1] | ||
IDLH (خطر عاجل)
|
Ca [N.D.][1] | ||
| مركبات ذا علاقة | |||
chloroethenes ذات العلاقة
|
dichloroethylenes, trichloroethylene, tetrachloroethylene, allyl chloride | ||
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |||
| مراجع الجدول | |||
كلوريد الڤاينيل، هو كلوريد عضوي صيغته الكيميائية H2C=CHCl ويسمى مونومر كلوريد الڤاينيل (VCM) أو إيثان الكلور. وهو مركب عديم اللون ومادة كيميائية صناعية هامة تستخدم بصفة أساسية لإنتاج پوليمر كلوريد پوليڤنيل (پي ڤي سي).[2] ينتج منه حوالي 13 بليون كيلوگرام سنوياً. ويعتبر كلوريد الڤاينيل من بين الپتروكيماويات العشرين الكبرى (كيماويات مشتقة من البترول) على مستوى الإنتاج العالمي.[بحاجة لمصدر] لا زالت الولايات المتحدة هي المنطقة الرئيسية لإنتاج كلوريد الڤاينيل في العالم بسبب انخفاض سعر إنتاج خام الكلور والإثيلين الخام بها. كما تعتبر الصين من أكبر مصنعي كلوريد الڤاينيل ووحدة من أكبر مستهلكيه في العالم.[3] كلوريد الڤاينيل هو غاز ذو رائحة ذكية. وهو عالي السمية، قابل للاشتعال ومسرطن. يمكن تشكله في البيئة عند تحلل عضيات التربة إلى محاليل مكلورة. ينطلق كلوريد الڤاينيل من الصناعات وبتكسر الكيماويات المكلورة يمكن أن يدخل الهواء وإلى موارد مياه الشرب. كلوريد الڤاينيل هو أحد الملوثات الشائعة الموجودة بالقرب من مدافن النفايات.[4] في السابق كان كلوريد الڤاينيل يستخدم كمبرد.[5]
الإنتاج
نظراً لأهمية هذا المونومير فقد تم إتباع كل الطرق في سبيل تصنيع هذا المركب وحالياً فإن المواد الأساسية الضرورية هي الكلور والإستيلين أو الإيتلين، ويمكن استخدام الكلور على شكل عنصري فهو يحضر لذلك من التحليل الكهربائي لكلوريدات المعادن القلوية أو على شكل حمض كلور الماء.
هناك أربع طرق اصطناع وهي :
أ- نزع كلوريد الهيدروجين من 2،1–ثناثي كلور الايتان تحت تأثير القلويات أو بالتحلل الحراري ويتم بدرجات حرارة عالية (450-500 °س) وبوجود وسيط من الحديد.
ب-بدءاً من الإستيلين : تتم بتفاعل HCl مع الإستيلين في الدرجة 150-200 °C وتتم في الحالة الغازية في الدرجة المذكورة وبوجود وسيط من كلور الزئبق أو في وسط مائي في الدرجة 20-25 °س وهذه الطريقة لا تعطي نواتج ثانوية.
ج- بدءاً من الإيتلين : وذلك بكلورة الإيتلين حيث نحصل على 2،1–ثناثي كلور الايتان وذلك في الدرجة (40-60 °س) وثلاثي كلور الحديد كحفاز، وبنزع HCl في درجة حرارة عالية يعطي ثنائي كلور الإيتان جزيء كلور الفينيل وذلك عند درجات حرارة عالية تتراوح بين (400-500 °س). بوجود أكسيد الألمنيوم والكربون النشط كحفاز.
ويمكن كلورة الاتلين مباشرة وذلك عند درجات حرارة عالية (500-600 °س).
د- طريقة الأكسدة الكلورية :ويتم هذا التفاعل عند درجة حرارة عالية بحدود 470-500 °س
الإنتاج من الإيثلين
| المادة الخام | الكمية المطلوبة |
|---|---|
| الإيثلين | 459 كگ |
| الكلور | 575 كگ |
| الأكسجين | 139 كگ |
| البخار | 250 كگ |
| غاز الوقود | 2.7 ك.ج |
الاستخدامات

Vinyl chloride, also called vinyl chloride monomer (VCM), is exclusively used as a precursor to PVC. Due to its toxic nature, vinyl chloride is not found in other products. Poly(vinyl chloride) (PVC) is very stable, storable and not toxic.[2]
Until 1974, vinyl chloride was used in aerosol spray propellant.[6] Vinyl chloride was briefly used as an inhalational anaesthetic, in a similar vein to ethyl chloride, though its toxicity limited this use.[7][8]
التخزين والنقل
Vinyl chloride is stored as a liquid. The presently accepted upper limit of safety as a health hazard is 500 ppm. Often, the storage containers for the product vinyl chloride are high capacity spheres. The spheres have an inside sphere and an outside sphere. Several inches of empty space separate the inside sphere from the outside sphere. This void area between the spheres is purged with an inert gas such as nitrogen. As the nitrogen purge gas exits the void space it passes through an analyzer that is designed to detect if any vinyl chloride is leaking from the internal sphere. If vinyl chloride starts to leak from the internal sphere or if a fire is detected on the outside of the sphere then the contents of the sphere are automatically dumped into an emergency underground storage container. Containers used for handling vinyl chloride at atmospheric temperature are always under pressure. Inhibited vinyl chloride may be stored at normal atmospheric conditions in suitable pressure vessel. Uninhibited vinyl chloride may be stored either under refrigeration or at normal atmospheric temperature in the absence of air or sunlight but only for a duration of a few days. If for longer periods, regular checks should be made for the presence of polymers.[9]
Transporting VCM presents the same risks as transporting other flammable gases such as propane, butane (LPG) or natural gas (for which the same safety regulations apply). The equipment used for VCM transport is specially designed to be impact and corrosion resistant.[10]
Fire and explosion hazard
In the U.S., OSHA lists vinyl chloride as a Class IA Flammable Liquid, with a National Fire Protection Association Flammability Rating of 4. Because of its low boiling point, liquid VCM will undergo flash evaporation (i.e., autorefrigerate) upon its release to atmospheric pressure. The portion vaporized will form a dense cloud (more than twice as heavy as the surrounding air). The risk of subsequent explosion or fire is significant. According to OSHA, the flash point of vinyl chloride is −78 °C (−108.4 °F).[11] Its flammable limits in air are: lower 3.6 volume% and upper 33.0 volume%. The explosive limits are: lower 4.0%, upper 22.05% by volume in air. Fire may release toxic hydrogen chloride (HCl) and carbon monoxide (CO).[12] VCM can polymerise rapidly due to heating and under the influence of air, light and contact with a catalyst, strong oxidisers and metals such as copper and aluminium, with fire or explosion hazard. As a gas mixed with air, VCM is a fire and explosion hazard. On standing, VCM can form peroxides, which may then explode. VCM will react with iron and steel in the presence of moisture.[5][13]
Health effects
Vinyl chloride finds its major application in the production of PVC. It is volatile, so the primary exposure is via inhalation, as opposed to the consumption of contaminated food or water, with occupational hazards being highest. Prior to 1974, workers were commonly exposed to 1,000 ppm vinyl chloride, causing "vinyl chloride illness" such as acroosteolysis and Raynaud's Phenomenon. The symptoms of vinyl chloride exposure are classified by ppm levels in ambient air with 4,000 ppm having a threshold effect.[14] The intensity of symptoms varies from acute (1,000–8,000 ppm), including dizziness, nausea, visual disturbances, headache, and ataxia, to chronic (above 12,000 ppm), including narcotic effect, cardiac arrhythmias, and fatal respiratory failure.[15] RADS (Reactive Airway Dysfunction Syndrome) may be caused by acute exposure to vinyl chloride.[16]
Vinyl chloride is a mutagen having clastogenic effects which affect lymphocyte chromosomal structure.[15][17] Vinyl chloride is a Group 1 human carcinogen posing elevated risks of rare angiosarcoma, brain and lung tumors, and malignant haematopoeitic lymphatic tumors.[18] Chronic exposure leads to common forms of respiratory failure (emphysema, pulmonary fibrosis) and focused hepatotoxicity (hepatomegaly, hepatic fibrosis). Continuous exposure can cause CNS depression including euphoria and disorientation. Decreased male libido, spontaneous abortion, and birth defects are known major reproductive defects associated with vinyl chloride.
Vinyl chloride can have acute dermal and ocular effects. Dermal exposure effects are thickening of skin, edema, decreased elasticity, local frostbites, blistering, and irritation.[15] The complete loss of skin elasticity expresses itself in Raynaud’s Phenomenon.[17]
Liver toxicity
The hepatotoxicity of vinyl chloride has long been established since the 1930s when the PVC industry was just in its infant stages. In the very first study about the dangers of vinyl chloride, published by Patty in 1930, it was disclosed that exposure of test animals to just a single short-term high dose of vinyl chloride caused liver damage.[19] In 1949, a Russian publication discussed the finding that vinyl chloride caused liver injury among workers.[20] In 1954, B.F. Goodrich Chemical stated that vinyl chloride caused liver injury upon short-term exposures. Almost nothing was known about its long-term effects. They also recommended long-term animal toxicology studies. The study noted that if a chemical did justify the cost of testing, and its ill-effects on workers and the public were known, the chemical should not be made.[21] In 1963, research paid for in part by Allied Chemical found liver damage in test animals from exposures below 500 parts per million (ppm).[22] Also in 1963, a Romanian researcher published findings of liver disease in vinyl chloride workers.[23] In 1968, Mutchler and Kramer, two Dow researchers, reported their finding that exposures as low as 300 ppm caused liver damage in vinyl chloride workers thus confirming earlier animal data in humans.[24] In a 1969 presentation given in Japan, P. L. Viola, a European researcher working for the European vinyl chloride industry, indicated, "every monomer used in V.C. manufacture is hazardous....various changes were found in bone and liver. Particularly, much more attention should be drawn to liver changes. The findings in rats at the concentration of 4 to 10 ppm are shown in pictures." In light of the finding of liver damage in rats from just 4–10 ppm of vinyl chloride exposure, Viola added that he "should like some precautions to be taken in the manufacturing plants polymerizing vinyl chloride, such as a reduction of the threshold limit value of monomer."[25]
Cancerous tumors
In 1970, Viola reported that test animals exposed to 30,000 ppm of vinyl chloride developed cancerous tumors. Viola began his research looking for the cause of liver and bone injuries found in vinyl chloride workers. Viola's findings in 1970 were a "red flag" to B.F. Goodrich and the industry.[26] In 1972, Maltoni, another Italian researcher for the European vinyl chloride industry, found liver tumors (including angiosarcoma) from vinyl chloride exposures as low as 250 ppm for four hours a day.[27]
In 1997 the U.S. Centers for Disease Control and Prevention (CDC) concluded that the development and acceptance by the PVC industry of a closed loop polymerization process in the late 1970s "almost completely eliminated worker exposures" and that "new cases of hepatic angiosarcoma in vinyl chloride polymerization workers have been virtually eliminated."[28]
The Houston Chronicle claimed in 1998 that the vinyl industry manipulated vinyl chloride studies to avoid liability for worker exposure and hid extensive and severe chemical spills in local communities.[29]
Environment pollution
According to the EPA, "vinyl chloride emissions from polyvinyl chloride (PVC), ethylene dichloride (EDC), and vinyl chloride monomer (VCM) plants cause or contribute to air pollution that may reasonably be anticipated to result in an increase in mortality or an increase in serious irreversible, or incapacitating reversible illness. Vinyl chloride is a known human carcinogen that causes a rare cancer of the liver."[30] EPA's 2001 updated Toxicological Profile and Summary Health Assessment for VCM in its Integrated Risk Information System (IRIS) database lowers EPA's previous risk factor estimate by a factor of 20 and concludes that "because of the consistent evidence for liver cancer in all the studies...and the weaker association for other sites, it is concluded that the liver is the most sensitive site, and protection against liver cancer will protect against possible cancer induction in other tissues."[31]
Microbial remediation
The bacteria species Nitrosomonas europaea can degrade a variety of halogenated compounds including trichloroethylene, and vinyl chloride.[32]
انظر أيضاً
المصادر
- ^ أ ب ت ث ج NIOSH Pocket Guide to Chemical Hazards 0658
- ^ أ ب خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةUllmann - ^ "Vinyl Chloride Monomer (VCM) - Chemical Economics Handbook (CEH) - IHS Markit". www.ihs.com. Retrieved 5 April 2018.
- ^ "http://www.dhs.wisconsin.gov/eh/chemfs/fs/vc.htm"
- ^ أ ب "http://www.npi.gov.au/resource/vinyl-chloride-monomer-vcm"
- ^ Markowitz, Gerald; Rosner, David (2013). Deceit and Denial: The Deadly Politics of Industrial Pollution. Berkeley, California Press: University of California Press. p. 185.[dead link]
- ^ Tamburro CH (1978). "Health effects of vinyl chloride". Texas Reports on Biology and Medicine. 37: 126–44, 146–51. PMID 572591.
- ^ Oster RH, Carr CJ (July 1947). "Anesthesia; narcosis with vinyl chloride". Anesthesiology. 8 (4): 359–61. doi:10.1097/00000542-194707000-00003. PMID 20255056. S2CID 73229069. Archived from the original on 2023-02-15. Retrieved 2023-02-15.
- ^ aseh.net[dead link]
- ^ "Vinyl Chloride Monomer (VCM) Production". Archived from the original on 2019-01-07.
- ^ "Aseh.net" (PDF).
- ^ "Occupational Safety and Health Guideline for Vinyl Chloride"1988."
- ^ "Vinyl chloride: health effects, incident management and toxicology". www.gov.uk. Retrieved 5 April 2018.
- ^ Harrison, Henrietta (2008). HPA Report Version 1. CHAP DHQ
- ^ أ ب ت International Programme on Chemical Safety (IPCS) (1999). Vinyl chloride. Environmental Health Criteria 215. WHO. Geneva.
- ^ UK Department for Environment, Food, and Rural Affairs (DEFRA) and Environment Agency (EA) (2004). "Contaminants in soil: Collation of toxicological data and intake values for humans. Vinyl chloride."
- ^ أ ب Agency for Toxic Substances and Disease Registry (July 2006). Toxicological profile for vinyl chloride. Atlanta, US: U.S. Department of Health and Human Services. Archived from the original. You must specify the date the archive was made using the
|archivedate=parameter. https://stacks.cdc.gov/view/cdc/6948/cdc_6948_DS1.pdf. - ^ International Agency for Research on Cancer (IARC). "Vinyl chloride, polyvinyl chloride, and vinyl chloride-vinyl acetate copolymers." Vol 19, 1979. IARC. "Vinyl chloride." Supplement 7, 1987. Lyon.
- ^ Patty, F. A.; Yant, W. P.; Waite, C. P. (1930). "Acute Response of Guinea Pigs to Vapors of Some New Commercial Organic Compounds: V. Vinyl Chloride". Public Health Reports (1896-1970) (in الإنجليزية). 45 (34): 1963. doi:10.2307/4579760.
- ^ Tribukh, S L et al. "Working Conditions and Measures for Their Improvement in Production and Use of Vinylchloride Plastics" (1949)
- ^ Wilson, Rex H et al. "Toxicology of Plastics and Rubber – Plastomers and Monomers." Reprinted from Industrial Medicine and Surgery. 23:11, 479–786. November 1954.
- ^ Lester, D.; Greenberg, L. A.; Adams, W. Robert (May 1963). "Effects of Single and Repeated Exposures of Humans and Rats to Vinyl Chloride". American Industrial Hygiene Association Journal (in الإنجليزية). 24 (3): 265–275. doi:10.1080/00028896309342963. ISSN 0002-8894.
- ^ Suciu, I.; Prodan, L.; Ilea, Elena; Păduraru, A.; Pascu, Livia (January 1975). "CLINICAL MANIFESTATIONS IN VINYL CHLORIDE POISONING". Annals of the New York Academy of Sciences (in الإنجليزية). 246: 53–69. doi:10.1111/j.1749-6632.1975.tb51080.x. ISSN 0077-8923.
- ^ Kramer, G.C., M.D. "The Correlation of Clinical and Environmental Measurements for Workers Exposed to Vinyl Chloride." The Dow Chemical Company. Midland Michigan.
- ^ Viola, P.L. "Pathology of Vinyl Chloride" International Congress on Occupational Health. Japan. 1969.
- ^ Viola, P L. "Carcinogenic Effect of Vinyl Chloride" Presented at the Tenth International Cancer Congress. Houston, Texas. May 22–29, 1970.
- ^ Maltoni, C. "Cancer Detection and Prevention" (1972) Presented at the Second International Symposium on Cancer Detection and Prevention. Bologna, April 9–12, 1973.
- ^ Epidemiologic Notes and Reports Angiosarcoma of the Liver Among Polyvinyl Chloride Workers – Kentucky. Centers for Disease Control and Prevention. 1997.
- ^ Jim Morris, "In Strictest Confidence. The chemical industry's secrets," Houston Chronicle. Part One: "Toxic Secrecy," June 28, 1998, pp. 1A, 24A–27A; Part Two: "High-Level Crime," June 29, 1998, pp. 1A, 8A, 9A; and Part Three: "Bane on the Bayou," July 26, 1998, pgs. 1A, 16A.
- ^ National Emission Standards for Hazardous Air Pollutants (NESHAP) for Vinyl Chloride Subpart F, OMB Control Number 2060-0071, EPA ICR Number 0186.09 (Federal Register: September 25, 2001 (Volume 66, Number 186))
- ^ EPA Toxicological Review of Vinyl Chloride in Support of Information on the IRIS. May 2000
- ^ "Home – Nitrosomonas europaea". genome.jgi-psf.org. Archived from the original on 3 July 2009. Retrieved 5 April 2018.
المراجع
- International Programme on Chemical Safety (IPCS) (1997). '"Vinyl chloride. Poisons Information Monograph. PIM 558. WHO. Geneva.
- National Poisons Information Service (NPIS) (2004). "Vinyl chloride." TOXBASE®.
- World Health Organisation (WHO) (2000). "Air quality guidelines for Europe." WHO Regional Publications, European Series, No. 91. 2nd edition. WHO Regional Office for Europe. Copenhagen.
- Hathaway G.J. and Proctor N.H. (2004). Chemical Hazards of the Workplace. 5th edition. John Wiley & Sons, New Jersey.
- Risk Assessment Information System (RAIS) (1993). "Toxicity summary for vinyl chloride. "Chemical Hazard Evaluation and Communication Group, Biomedical and Environmental Information Analysis Section, Health and Safety Research Division.
قراءات إضافية
- "Medicine: The Plastic Peril". Time. May 13, 1974. Retrieved 2 July 2010.
وصلات خارجية
- Articles with dead external links from July 2021
- Articles with dead external links from February 2023
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Short description is different from Wikidata
- مقالات ذات عبارات بحاجة لمصادر
- كيماويات سلعية
- ذيفان الكبد
- كلوريدات عضوية
- مركبات الڤاينيل
- مسرطنات المجموعة 1 حسب تصنيف الوكالة الدولية لأبحاث السرطان
- ملوثات الهواء الخطرة
- مونومرات