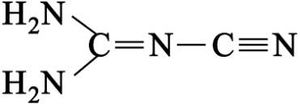سياناميد

| |||
|
| |||
| الأسماء | |||
|---|---|---|---|
| اسم أيوپاكs
Cyanamide,
aminomethanenitrile | |||
| أسماء أخرى
Amidocyanogen, carbamonitrile, carbimide, carbodiimide, cyanoamine, cyanoazane, N-cyanoamine, cyanogenamide, cyanogen amide, cyanogen nitride, diiminomethane, hydrogen cyanamide, methanediimine
| |||
| المُعرِّفات | |||
| رقم CAS | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.006.358 | ||
| رقم EC |
| ||
| مرجع Gmelin | 784 | ||
| KEGG | |||
PubChem CID
|
|||
| رقم RTECS |
| ||
| UNII | |||
| UN number | 2811 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| الخصائص | |||
| الصيغة الجزيئية | CH2N2 | ||
| كتلة مولية | 42.040 g/mol | ||
| المظهر | Crystalline solid | ||
| الكثافة | 1.28 g/cm3 | ||
| نقطة الانصهار | |||
| نقطة الغليان | |||
| قابلية الذوبان في الماء | 85 g/100 ml (25 °C) | ||
| قابلية الذوبان في organic solvents | soluble | ||
| log P | -0.82[1] | ||
| المخاطر | |||
| صفحة بيانات السلامة | ICSC 0424 | ||
| ن.م.ع. مخطط تصويري |    
| ||
| ن.م.ع. كلمة الاشارة | Danger | ||
| H301, H311, H314, H317, H351, H361, H373, H412 | |||
| P201, P202, P260, P261, P264, P270, P272, P273, P280, P281, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P310, P312, P314, P321, P322, P330, P333+P313, P361, P363, P405, P501 | |||
| NFPA 704 (معيـَّن النار) | |||
| نقطة الوميض | 141 °C (286 °F; 414 K) | ||
| حدود التعرض الصحية بالولايات المتحدة (NIOSH): | |||
PEL (المسموح)
|
none[2] | ||
REL (الموصى به)
|
TWA 2 mg/m3 | ||
IDLH (خطر عاجل)
|
N.D.[2] | ||
| مركبات ذا علاقة | |||
مركـّبات ذات علاقة
|
Calcium cyanamide | ||
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |||
| مراجع الجدول | |||
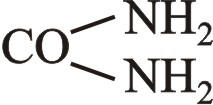
سياناميد cyanamide هو مركب عضوي صيغته CN2H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol-deterrent drug. The molecule features a nitrile group attached to an amino group. Derivatives of this compound are also referred to as cyanamides, the most common being calcium cyanamide (CaCN2).[3]
وهو مركَّب حمضي، وهو أميد حمض السيان HO-C≡N (أي استُبدلت NH2 في الحمض بـ OH). يشكّل إبراً لا لون لها ذات بنية معينيّة مستقيمة orthorhombique تتميّع عند تشرّبها الماء. يسمى أيضاً بلا ماء البولة urée anhydride. درجة انصهاره 44 ْس، ودرجة غليانه 410 ْس. يتميز طيفه تحت الأحمر بوجود قمة ثنائية عالية الشدة تراوح بين 2225 و2260سم-1 في حين يُظهر طيفه فوق البنفسجي امتصاصاً ضعيفاً عند طول الموجة 230 نانومتر.
Tautomers والتكثف الذاتي
Containing both a nucleophilic and electrophilic site within the same molecule, cyanamide undergoes various reactions with itself. Cyanamide exists as two tautomers, one with the connectivity N≡C–NH2 and the other with the formula HN=C=NH ("carbodiimide" tautomer). The N≡C–NH2 form dominates, but in a few reactions (e.g. silylation) the diimide form appears to be important.[3]
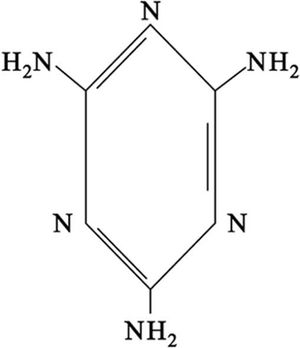
Cyanamide dimerizes to give 2-cyanoguanidine (dicyandiamide). This dimerization is disfavored by acids and is inhibited by low temperatures. The cyclic trimer is called melamine.[3]
تحضير السياناميد
يحضَّر السياناميد مخبرياً من البولة
بانتزاع الماء منها déshydration بتفاعله مع خماسي أكسيد ثنائي الفسفور P2O5. أما صناعياً فيحضَّر بتفاعل الكلس مع الكربون إذ يتأكـسـد كربيد الكالسيوم CaC2 الذي يخضع بدوره لتيار من غاز الآزوت عند درجة حرارة تراوح بين 1000 و1100 ْس فيتحول إلى سياناميد الكلسيوم CaNC≡N الذي تؤدي حلمهته بعد ذلك إلى تشكيل السياناميد.
التفاعلات والإستخدامات
يستخدم كل من السـياناميد وسـياناميد الكلسيوم أسمدة ومبيدات للأعشاب الضارة، وله أهمية خاصة في الصناعة لاستعماله مادة أولية لصنعثنائي السـياناميد C2H4N4 وهو متماثره الثنائي dimère ثم ثلاثي السياناميد C3H6N6 وهو متماثره الثلاثي trimère ويعرف باسم الميلامين mélamine. ويقوم الميلامين بدور مهم في الصناعات البلاستيكية، فهو المادة الأولية في صناعة لدائن الميلامين ـ فورم ألدهيد.[4]
Cyanamide can be regarded as a functional single carbon fragment which can react as an electrophile or nucleophile. The main reaction exhibited by cyanamide involves additions of compounds containing an acidic proton. Water, hydrogen sulfide, and hydrogen selenide react with cyanamide to give urea, thiourea, and selenourea, respectively:
- H2NCN + H2E → H2NC(E)NH2 (E = O, S, Se)
In this way, cyanamide behaves as a dehydration agent and thus can induce condensation reactions. Alcohols, thiols, and amines react analogously to give alkylisoureas, "pseudothioureas", and guanidines. The anti-ulcer drug cimetidine is generated using such reactivity. Related reactions exploit the bifunctionality of cyanamide to give heterocycles, and this latter reactivity is the basis of several pharmaceutical syntheses such as the aminopyrimidine imatinib) and agrichemicals Amitrol and hexazinone. The hair-loss treatment minoxidil and the anthelmintics albendazole, flubendazole, and mebendazole feature 2-aminoimidazole substructures derived from cyanamide.[3] Cyanamide is also used in the synthesis of other pharmaceutical drugs including tirapazamine, etravirine, revaprazan, and dasantafil.
The cyanamide anion has the character of a pseudo chalcogen, cyanamide can therefore be regarded as analogue to water or hydrogen sulfide.
A convenient method for the preparation of secondary amines which are not contaminated with primary or tertiary amines is the reaction of cyanamide with alkyl halides to N,N-dialkylcyanamides which can easily be hydrolyzed to dialkylamines and then decarboxylated.[5] Cyanamide adds itself in the presence of N-bromosuccinimide to olefinic double bonds. The addition product is converted by bases to N-Cyanaziridine,[6] cyclized in the presence of acids to imidazolines, which can be further reacted to vicinal diamines by alkaline cleavage.[7]
Cyanamide is also a versatile synthetic building block for heterocycles: it forms 2-aminobenzimidazole with 1,2-diaminobenzene[8] and it forms with the readily available cyclic enamine 4-(1-cyclohexenyl)morpholine[9] and with elemental sulfur a 2-aminothiazole in good yields.[10]
Sodium dicyanamide is available in good yield and high purity from cyanamid and cyanogen chloride,[11][12] which is suitable as an intermediate for the synthesis of active pharmaceutical ingredients.[13] A guanidino group is introduced by reaction of cyanamide with sarcosine In the industrial synthesis of creatine:[14]
This synthesis route mostly avoids problematic impurities like chloroacetic acid, iminodiacetic acid, or dihydrotriazine that occur in other routes. The physiological precursor guanidinoacetic is obtained analogously by reacting cyanamide with glycine.
Methods to stabilize cyanamidefmel make it available on an industrial scale. Due to the strong affinity towards self-condensation in alkaline media (see above) solutions of cyanamide are stabilized by the addition of 0.5 wt% of monosodium phosphate as buffer. Solid cyanamide is produced by careful evaporation of the solvent and subsequent addition of a hydrolysis-labile ester of formic acid. The ester absorbs traces of moisture (suppression of urea formation), neutralizes alkalinity (ammonia) and continually releases small amounts of formic acid.[15]
الاستخدام الزراعي
Cyanamide, under the trade name Dormex, is a common agricultural rest-breaking agent applied in spring to stimulate uniform opening of buds, early foliation and bloom. Cyanamide can effectively compensate for the moderate lack of chilling units accumulated in the previous autumn and save the harvest that would otherwise be lost. It is particularly effective for woody plants such as blueberries, grapes, apples, peaches and kiwifruit. Most recently the product was approved for use on almonds and pistachios in the USA. Overdosage, high concentration and error in timing of application can damage the buds (especially of peach trees).[16] Growers may avoid damage by applying 30 days prior to bud break according to the label.
A 50% aqueous solution of cyanamide is also used as a biocide (disinfectant) particularly in pig farming, because it effectively kills salmonella and shigella and fights flies in all stages of development.[17]
الجوانب البيئية
Cyanamide degrades via hydrolysis to urea, an excellent fertilizer. Fungi, like Myrothecium verrucaria, accelerate this process utilizing the enzyme cyanamide hydratase.[18]
مجموعة السياناميد الوظيفية
Cyanamide is the name for a functional group with the formula NCNRR' where R and R' can be a variety of groups. These compounds are called cyanamides. One example is naphthylcyanamide, C10H7N(H)CN[19] Some cyanamides are prepared by alkylation of calcium cyanamide. Others, such as the naphthyl derivative, are produced indirectly.[11]
السياناميد في الفضاء
Due to its high permanent dipole moment (i.e., 4.32 ± 0.08 D),[20] cyanamide was detected by spectral emissions coming from the Sgr B2 molecular cloud (T < 100 K) through its microwave transitions as the first known interstellar molecule containing the NCN frame.[21]
السلامة
It is used as an alcohol-deterrent drug in Canada, Europe, and Japan.[3]
Cyanamide has a modest toxicity in humans.[22] Workplace exposure to hydrogen cyanamide sprays or exposure in people living in the vicinity of spraying have been reported as causing respiratory irritation, contact dermatitis, headache, and gastrointestinal symptoms of nausea, vomiting, or diarrhea.[22]
انظر أيضاً
المصادر
- ^ "Cyanamide_msds".
- ^ أ ب NIOSH Pocket Guide to Chemical Hazards 0160
- ^ أ ب ت ث ج Thomas Güthner; Bernd Mertschenk (2006). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_139.pub2.
{{cite encyclopedia}}: Cite has empty unknown parameter:|authors=(help) - ^ السياناميد، الموسوعة العربية
- ^ Jonczyk A, Ochal Z, Makosza M (1978). "Reactions of Organic Anions; LXXXV1. Catalytic Two-Phase Alkylation of Cyanamide". Synthesis. 1978 (12): 882–883. doi:10.1055/s-1978-24922.
- ^ Ponsold K, Ihn W (1970). "Die Addition von cyanamid und Halogen an Olefine ein neues Verfahren zur Darstellung von vic.-Halogencyanaminen und Aziridinen". Tetrahedron Lett. 11 (13): 1125–1128. doi:10.1016/S0040-4039(01)97925-0. PMID 5439242.
- ^ Kohn, Harold; Jung, Sang Hun (1983). "New stereoselective method for the preparation of vicinal diamines from olefins and cyanamide". Journal of the American Chemical Society. 105 (12): 4106–4108. doi:10.1021/ja00350a068..
- ^ Weiss, Stefan; Michaud, Horst; Prietzel, Horst; Krommer, Helmut (1973). "A New, Simple Synthesis of 2-Aminobenzimidazole". Angewandte Chemie International Edition in English. 12 (10): 841. doi:10.1002/anie.197308411..
- ^ S. Hünig, E. Lücke, and W. Brenninger (1961). "1-Morpholino-1-Cyclohexene". Organic Syntheses: 65. doi:10.15227/orgsyn.041.0065
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Gewald, K.; Spies, H.; Mayer, R. (1970). "Zur Reaktion von Enaminen mit Schwefel und Cyanamid" [On the Reaction of Enamines with Sulfur and Cyanamide]. Journal für Praktische Chemie. 312 (5): 776–779. doi:10.1002/prac.19703120507..
- ^ أ ب E. B. Vliet (1925). "Diallylcyanamide". Organic Syntheses. 5: 45. doi:10.15227/orgsyn.005.0045.
- ^ Verfahren zur Herstellung von Natrium-Dicyanamid, veröffentlicht am 10. August 2000, Anmelder: SKW Trostberg AG.
- ^ "Sodium dicyanamide (Na-dicyanamide)". lonza.com. Archived from the original on 2013-05-23. Retrieved 2019-07-01.
- ^ Deutsche Offenlegungsschrift DE-OS 10 2006 016 227 A1, Offenlegungsdatum: 11. Oktober 2007, Anmelder: Degussa GmbH.
- ^ Wehrstedt, Klaus-Dieter; Wildner, Werner; Güthner, Thomas; Holzrichter, Klaus; Mertschenk, Bernd; Ulrich, Armin (2009-10-30). "Safe transport of cyanamide". Journal of Hazardous Materials. 170 (2–3): 829–835. doi:10.1016/j.jhazmat.2009.05.043. ISSN 0304-3894. PMID 19505756.
- ^ Powell, A. (1999). "Action Program for Dormex Application on Peaches". Auburn University. Archived from the original on 2018-06-20.
- ^ "ALZOGUR®". AlzChem (in الألمانية). Retrieved 2019-07-01.
- ^ Stransky H, Amberger A (1973). "Isolierung und eigenschaften einer Cyanamid-hydratase (E.C.-Gruppe 4. 2.1.) aus Myrothecium verrucaria Alb. u. Schw" [Isolation and properties of a cyanamide hydratase (EC 4.2.1) from Myrothecium verrucaria]. Z. Pflanzenphysiol. 70: 74–87. doi:10.1016/S0044-328X(73)80049-2.
- ^ Homer W. J. Cressman (1947). "N-Methyl-1-Naphthylcyanamide". Organic Syntheses. 27: 56. doi:10.15227/orgsyn.027.0056.
- ^ Tyler, J.K.; Sheridan, J.; Costain, C.C. (August 1972). "The microwave spectra of cyanamide". Journal of Molecular Spectroscopy. 43 (2): 248–261. doi:10.1016/0022-2852(72)90021-5.
- ^ Turner, B. E.; Liszt, H. S.; Kaifu, N.; Kisliakov, A. G. (November 1975). "Microwave detection of interstellar cyanamide". The Astrophysical Journal. 201: L149. Bibcode:1975ApJ...201L.149T. doi:10.1086/181963.
- ^ أ ب Schep L, Temple W, Beasley M (January 2009). "The adverse effects of hydrogen cyanamide on human health: an evaluation of inquiries to the New Zealand National Poisons Centre". Clinical Toxicology. Philadelphia, PA. 47 (1): 58–60. doi:10.1080/15563650802459254. PMID 18951270. S2CID 6961576.
وصلات خارجية
- CS1 الألمانية-language sources (de)
- Short description is different from Wikidata
- Articles with hatnote templates targeting a nonexistent page
- ECHA InfoCard ID from Wikidata
- GHS warnings
- Articles containing unverified chemical infoboxes
- Chembox image size set
- سياناميد
- الأسمدة
- سلامة الأغذية
- مركبات الكربون غير العضوية
- نتروجين
- أيض النيتروجين