دواء غفل
الغفل أو (باللاتينية: placebo) هو عبارة عن مادة تأخذ شكل الدواء ولكنها لا تحتوي علي أية عناصر فعالة ولذلك فأن أي تغيير يحدث للمريض فإنه يرجع للعوامل النفسية فقط مثل الإيحاء والتوقع أو السير الطبيعي للمرض.
وقد اهتم علماء النفس بدراسة الأفراد الذين يؤثر عليهم الدواء الغفل وأشاروا أنهم أميل للاتصاف بالقلق النفسي أو الإنطواء.
ويستخدم الدواء الغفل أيضا في الدراسة التجريبية للأدوية؛ فمثلا يكون هناك مجموعتان من الأفراد المجموعة الأولى تتناول الدواء الحقيقي والمجموعة الثانية تتناول الدواء الغفل ولذك لمعرفة مدى التأثير الحقيقي لهذا الدواء على متناوليه. ,يستخدم الغفل في التجارب السريرية لتعمية الناس عن العلاج الذي يتلقونه، ولضمان التعمية الصحيحة، يجب أن يكون الغفل غير قابلاً للتمييز عن الدواء الفعّال، ففي التجارب السريرية الدوائية مثلاً، يجب أن تكون حبوب الغفل مطابقةً تماما لحبوب الدواء الفعال من حيث الشكل والحجم واللون والوزن. هذا ويستخدم الغفل أحياناً بشكل علاجي حيث يفيد نفسياً.
إن تأثير الغفل هو ظاهرة ممكن أن تؤدي لتخفيف أعراض المرض بوساطة علاج غير فعال، لمجرد أن المريض يريد أو يعتقد بأن هذا العلاج مفيد وفعال. ويعتبر مصطلح "تأثير الغفل" حديث فعلياً ويعبر عن التأثيرات المختلفة التي تجعل المرضى يعيدون التفكير بأعراض مرضهم.
حيث أن تأثير الغفل هو استجابة نفسية (سيكولوجية) للعلاج. والأمراض الجسدية لا تتحسن بتأثير الغفل. فالغفل لا يشفي المرض - كما يعتقد كثير من ا لناس - بل ما يحدث هو ملاحظة تحسن الأعراض والذي يميز تأثير الغفل.
ويجب أن يلاحظ أن جميع الأدوية تزيد من تأثير الغفل. وتعتبر المعالجات فعالة إن كان لها تأثير أقوى وأكثر فعالية من التأثيرات الناتجة عن الغفل.
تعريفات
The American Society of Pain Management Nursing define a placebo as "any sham medication or procedure designed to be void of any known therapeutic value".[2]
In a clinical trial, a placebo response is the measured response of subjects to a placebo; the placebo effect is the difference between that response and no treatment.[3] It is also part of the recorded response to any active medical intervention.[4]
Any measurable placebo effect is termed either objective (e.g. lowered blood pressure) or subjective (e.g. a lowered perception of pain).[2]
التأثيرات
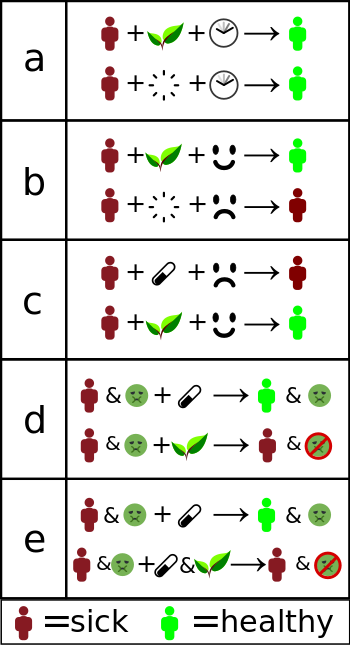
a) Misinterpreted natural course – the individual gets better without treatment.
b) Placebo effect or false treatment effect – an individual receives "alternative therapy" and is convinced it will help. The conviction makes them more likely to get better.
c) Nocebo effect – an individual is convinced that standard treatment will not work, and that alternative therapies will work. This decreases the likelihood standard treatment will work, while the placebo effect of the "alternative" remains.
d) No adverse effects – Standard treatment is replaced with "alternative" treatment, getting rid of adverse effects, but also of improvement.
e) Interference – Standard treatment is "complemented" with something that interferes with its effect. This can both cause worse effect, but also decreased (or even increased) side effects, which may be interpreted as "helping". Researchers, such as epidemiologists, clinical statisticians and pharmacologists, use clinical trials to reveal such effects, allowing physicians to offer a therapeutic solution best known to work. "Alternative treatments" often refuse to use trials or make it deliberately hard to do so.
Placebos can improve patient-reported outcomes such as pain and nausea.[5] This effect is unpredictable and hard to measure, even in the best conducted trials.[5] For example, if used to treat insomnia, placebos can cause patients to perceive that they are sleeping better, but do not improve objective measurements of sleep onset latency.[6] A 2001 Cochrane Collaboration meta-analysis of the placebo effect looked at trials in 40 different medical conditions, and concluded the only one where it had been shown to have a significant effect was for pain.[7]
By contrast, placebos do not appear to affect the actual diseases, or outcomes that are not dependent on a patient's perception.[5] One exception to the latter is Parkinson's disease, where recent research has linked placebo interventions to improved motor functions.[8][9][10]
Measuring the extent of the placebo effect is difficult due to confounding factors.[11] For example, a patient may feel better after taking a placebo due to regression to the mean (i.e. a natural recovery or change in symptoms).[12][13][14] It is harder still to tell the difference between the placebo effect and the effects of response bias, observer bias and other flaws in trial methodology, as a trial comparing placebo treatment and no treatment will not be a blinded experiment.[5][12] In their 2010 meta-analysis of the placebo effect, Asbjørn Hróbjartsson and Peter C. Gøtzsche argue that "even if there were no true effect of placebo, one would expect to record differences between placebo and no-treatment groups due to bias associated with lack of blinding."[5]
Hróbjartsson and Gøtzsche concluded that their study "did not find that placebo interventions have important clinical effects in general."[5] Jeremy Howick has argued that combining so many varied studies to produce a single average might obscure that "some placebos for some things could be quite effective."[15] To demonstrate this, he participated in a systematic review comparing active treatments and placebos using a similar method, which generated a clearly misleading conclusion that there is "no difference between treatment and placebo effects".[16][15]
العوامل المؤثرة على قوة تأثير الدواء الغفل

A review published in JAMA Psychiatry found that, in trials of antipsychotic medications, the change in response to receiving a placebo had increased significantly between 1960 and 2013. The review's authors identified several factors that could be responsible for this change, including inflation of baseline scores and enrollment of fewer severely ill patients.[19] Another analysis published in Pain in 2015 found that placebo responses had increased considerably in neuropathic pain clinical trials conducted in the United States from 1990 to 2013. The researchers suggested that this may be because such trials have "increased in study size and length" during this time period.[20]
Children seem to have greater response than adults to placebos.[21]
Some studies have investigated the use of placebos where the patient is fully aware that the treatment is inert, known as an open-label placebo.[22] A meta-analysis found some evidence that open-label placebos may have positive effects in comparison to no treatment,[23] which may open new avenues for treatments,[22] but noted the trials were done with a small number of participants and hence should be interpreted with "caution" until further better controlled trials are conducted.[23][22]
الأعراض والأمراض
A 2010 Cochrane Collaboration review suggests that placebo effects are apparent only in subjective, continuous measures, and in the treatment of pain and related conditions.[5]
الأم
Placebos are believed to be capable of altering a person's perception of pain. "A person might reinterpret a sharp pain as uncomfortable tingling."[24]
One way in which the magnitude of placebo analgesia can be measured is by conducting "open/hidden" studies, in which some patients receive an analgesic and are informed that they will be receiving it (open), while others are administered the same drug without their knowledge (hidden). Such studies have found that analgesics are considerably more effective when the patient knows they are receiving them.[25]
الاكتئاب
In 2008, a controversial meta-analysis led by psychologist Irving Kirsch, analyzing data from the FDA, concluded that 82% of the response to antidepressants was accounted for by placebos.[26] However, there are serious doubts about the used methods and the interpretation of the results, especially the use of 0.5 as cut-off point for the effect-size.[27] A complete reanalysis and recalculation based on the same FDA data discovered that the Kirsch study suffered from "important flaws in the calculations". The authors concluded that although a large percentage of the placebo response was due to expectancy, this was not true for the active drug. Besides confirming drug effectiveness, they found that the drug effect was not related to depression severity.[28]
Another meta-analysis found that 79% of depressed patients receiving placebo remained well (for 12 weeks after an initial 6–8 weeks of successful therapy) compared to 93% of those receiving antidepressants. In the continuation phase however, patients on placebo relapsed significantly more often than patients on antidepressants.[29]
الآثار السلبية
A phenomenon opposite to the placebo effect has also been observed. When an inactive substance or treatment is administered to a recipient who has an expectation of it having a negative impact, this intervention is known as a nocebo (Latin nocebo = "I shall harm").[30] A nocebo effect occurs when the recipient of an inert substance reports a negative effect or a worsening of symptoms, with the outcome resulting not from the substance itself, but from negative expectations about the treatment.[31][32]
Another negative consequence is that placebos can cause side-effects associated with real treatment.[33] Failure to minimise nocebo side-effects in clinical trials and clinical practice raises a number of recently explored ethical issues. [34]
Withdrawal symptoms can also occur after placebo treatment. This was found, for example, after the discontinuation of the Women's Health Initiative study of hormone replacement therapy for menopause. Women had been on placebo for an average of 5.7 years. Moderate or severe withdrawal symptoms were reported by 4.8% of those on placebo compared to 21.3% of those on hormone replacement.[35]
الأخلاقيات
في التجارب البحثية
في الممارسة الطبية
الآليات
Expectation plays a clear role. A placebo presented as a stimulant may trigger an effect on heart rhythm and blood pressure, but when administered as a depressant, the opposite effect.[36]
علم النفس
In psychology, the two main hypotheses of placebo effect are expectancy theory and classical conditioning.[37]
In 1985, Irving Kirsch hypothesized that placebo effects are produced by the self-fulfilling effects of response expectancies, in which the belief that one will feel different leads a person to actually feel different.[38] According to this theory, the belief that one has received an active treatment can produce the subjective changes thought to be produced by the real treatment. Similarly, the appearance of effect can result from classical conditioning, wherein a placebo and an actual stimulus are used simultaneously until the placebo is associated with the effect from the actual stimulus.[39] Both conditioning and expectations play a role in placebo effect,[37] and make different kinds of contribution. Conditioning has a longer-lasting effect,[40] and can affect earlier stages of information processing.[41] Those who think a treatment will work display a stronger placebo effect than those who do not, as evidenced by a study of acupuncture.[42]
Additionally, motivation may contribute to the placebo effect. The active goals of an individual changes their somatic experience by altering the detection and interpretation of expectation-congruent symptoms, and by changing the behavioral strategies a person pursues.[43] Motivation may link to the meaning through which people experience illness and treatment. Such meaning is derived from the culture in which they live and which informs them about the nature of illness and how it responds to treatment.
التسكين الوهمي
Functional imaging upon placebo analgesia suggests links to the activation, and increased functional correlation between this activation, in the anterior cingulate, prefrontal, orbitofrontal and insular cortices, nucleus accumbens, amygdala, the brainstem periaqueductal gray matter,[44][45] and the spinal cord.[46][47][48]
It has been known that placebo analgesia depends upon the release in the brain of endogenous opioids since 1978.[49] Such analgesic placebos activation changes processing lower down in the brain by enhancing the descending inhibition through the periaqueductal gray on spinal nociceptive reflexes, while the expectations of anti-analgesic nocebos acts in the opposite way to block this.[46]
Functional imaging upon placebo analgesia has been summarized as showing that the placebo response is "mediated by "top-down" processes dependent on frontal cortical areas that generate and maintain cognitive expectancies. Dopaminergic reward pathways may underlie these expectancies".[50] "Diseases lacking major 'top-down' or cortically based regulation may be less prone to placebo-related improvement".[51]
المخ والجسم
In conditioning, a neutral stimulus saccharin is paired in a drink with an agent that produces an unconditioned response. For example, that agent might be cyclophosphamide, which causes immunosuppression. After learning this pairing, the taste of saccharin by itself is able to cause immunosuppression, as a new conditioned response via neural top-down control.[52] Such conditioning has been found to affect a diverse variety of not just basic physiological processes in the immune system but ones such as serum iron levels, oxidative DNA damage levels, and insulin secretion. Recent reviews have argued that the placebo effect is due to top-down control by the brain for immunity[53] and pain.[54] Pacheco-López and colleagues have raised the possibility of "neocortical-sympathetic-immune axis providing neuroanatomical substrates that might explain the link between placebo/conditioned and placebo/expectation responses."[53] There has also been research aiming to understand underlying neurobiological mechanisms of action in pain relief, immunosuppression, Parkinson's disease and depression.[55]
Dopaminergic pathways have been implicated in the placebo response in pain and depression.[56]
العوامل الخارجية
Placebo-controlled studies, as well as studies of the placebo effect itself, often fail to adequately identify confounding factors.[24][57][58] False impressions of placebo effects are caused by many factors including:[24][12][58][37][57]
- Regression to the mean (natural recovery or fluctuation of symptoms)
- Additional treatments
- Response bias from subjects, including scaling bias, answers of politeness, experimental subordination, conditioned answers;
- Reporting bias from experimenters, including misjudgment and irrelevant response variables.
- Non-inert ingredients of the placebo medication having an unintended physical effect
التاريخ
انظر أيضاً
المصادر
- ^ Lanotte M, Lopiano L, Torre E, Bergamasco B, Colloca L, Benedetti F. (2005) Expectation enhances autonomic responses to stimulation of the human subthalamic limbic region. Brain Behav Immun. 19:500-9. PubMed
- ^ أ ب Arnstein P, Broglio K, Wuhrman E, Kean MB (2011). "Use of placebos in pain management" (PDF). Pain Manag Nurs (Position Statement of the American Society for Pain Management Nursing). 12 (4): 225–9. doi:10.1016/j.pmn.2010.10.033. PMID 22117754.
- ^ Chaplin S (2006). "The placebo response: an important part of treatment". Prescriber. 17 (5): 16–22. doi:10.1002/psb.344.
- ^ Eccles R (2002). "The powerful placebo in cough studies?". Pulmonary Pharmacology & Therapeutics. 15 (3): 303–8. doi:10.1006/pupt.2002.0364. PMID 12099783.
- ^ أ ب ت ث ج ح خ Hróbjartsson A, Gøtzsche PC (January 2010). Hróbjartsson A (ed.). "Placebo interventions for all clinical conditions" (PDF). The Cochrane Database of Systematic Reviews. 106 (1): CD003974. doi:10.1002/14651858.CD003974.pub3. PMC 7156905. PMID 20091554.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Yeung V, Sharpe L, Glozier N, Hackett ML, Colagiuri B (April 2018). "A systematic review and meta-analysis of placebo versus no treatment for insomnia symptoms". Sleep Medicine Reviews. 38: 17–27. doi:10.1016/j.smrv.2017.03.006. PMID 28554719.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةHrob2001 - ^ Quattrone, Aldo; Barbagallo, Gaetano; Cerasa, Antonio; Stoessl, A. Jon (August 2018). "Neurobiology of placebo effect in Parkinson's disease: What we have learned and where we are going". Movement Disorders. 33 (8): 1213–1227. doi:10.1002/mds.27438. ISSN 1531-8257. PMID 30230624.
- ^ Gross, Liza (February 2017). "Putting placebos to the test". PLOS Biology. 15 (2): e2001998. doi:10.1371/journal.pbio.2001998. ISSN 1545-7885. PMC 5319646. PMID 28222121.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Enck, Paul; Bingel, Ulrike; Schedlowski, Manfred; Rief, Winfried (March 2013). "The placebo response in medicine: minimize, maximize or personalize?". Nature Reviews Drug Discovery (in الإنجليزية). 12 (3): 191–204. doi:10.1038/nrd3923. ISSN 1474-1776. PMID 23449306.
- ^ Hróbjartsson A, Gøtzsche PC (August 2004). "Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment". Journal of Internal Medicine. 256 (2): 91–100. doi:10.1111/j.1365-2796.2004.01355.x. PMID 15257721. Gøtzsche's biographical article has further references related to this work.
- ^ أ ب ت Kienle GS, Kiene H (December 1997). "The powerful placebo effect: fact or fiction?". Journal of Clinical Epidemiology. 50 (12): 1311–8. doi:10.1016/s0895-4356(97)00203-5. PMID 9449934.
- ^ McDonald CJ, Mazzuca SA, McCabe GP (1983). "How much of the placebo 'effect' is really statistical regression?". Statistics in Medicine. 2 (4): 417–27. doi:10.1002/sim.4780020401. PMID 6369471.
- ^ Barnett AG, van der Pols JC, Dobson AJ (February 2005). "Regression to the mean: what it is and how to deal with it". International Journal of Epidemiology. 34 (1): 215–20. doi:10.1093/ije/dyh299. PMID 15333621.
- ^ أ ب Hutchinson P, Moerman D (October 2018). "The Meaning Response, "Placebo" and Method". Perspectives in Biology and Medicine. 15 (2): 361–378. doi:10.1353/pbm.2018.0049. PMID 30293975.
- ^ Howick J, Friedemann C, Tsakok M, Watson R, Tsakok T, Thomas J, Perera R, Fleming S, Heneghan C (May 2013). "Are Treatments More Effective than Placebos? A Systematic Review and Meta-Analysis". PLOS One. 11 (1): e62599. Bibcode:2013PLoSO...862599H. doi:10.1371/journal.pone.0062599. PMC 3655171. PMID 23690944.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Wright, P (2003). "Obituary: Louis Lasagna". The Lancet. Elsevier. 362 (9393): 1423. doi:10.1016/S0140-6736(03)14640-5. Retrieved 19 April 2009.
- ^ Lasagna, Louis (1964). "Hippocratic Oath—Modern Version". WGBH Educational Foundation for PBS and NOVA Online. Retrieved 7 November 2001.
- ^ Rutherford BR, Pott E, Tandler JM, Wall MM, Roose SP, Lieberman JA (December 2014). "Placebo response in antipsychotic clinical trials: a meta-analysis". JAMA Psychiatry. 71 (12): 1409–21. doi:10.1001/jamapsychiatry.2014.1319. PMC 4256120. PMID 25321611.
- ^ Tuttle AH, Tohyama S, Ramsay T, Kimmelman J, Schweinhardt P, Bennett GJ, Mogil JS (December 2015). "Increasing placebo responses over time in U.S. clinical trials of neuropathic pain". Pain. 156 (12): 2616–26. doi:10.1097/j.pain.0000000000000333. PMID 26307858.
{{cite journal}}: Unknown parameter|lay-url=ignored (help) - ^ Rheims S, Cucherat M, Arzimanoglou A, Ryvlin P (August 2008). Klassen T (ed.). "Greater response to placebo in children than in adults: a systematic review and meta-analysis in drug-resistant partial epilepsy". PLOS Medicine. 5 (8): e166. doi:10.1371/journal.pmed.0050166. PMC 2504483. PMID 18700812.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ أ ب ت Blease, CR; Bernstein, MH; Locher, C (26 June 2019). "Open-label placebo clinical trials: is it the rationale, the interaction or the pill?". BMJ Evidence-based Medicine (Review): bmjebm–2019–111209. doi:10.1136/bmjebm-2019-111209. PMC 6930978. PMID 31243047.
- ^ أ ب Charlesworth JE, Petkovic G, Kelley JM, Hunter M, Onakpoya I, Roberts N, Miller FG, Howick J (May 2017). "Effects of placebos without deception compared with no treatment: A systematic review and meta-analysis" (PDF). Journal of Evidence-Based Medicine (Systematic review and meta-analysis). 10 (2): 97–107. doi:10.1111/jebm.12251. PMID 28452193.
- ^ أ ب ت "Placebo Effect". American Cancer Society. 10 April 2015.
- ^ Price DD, Finniss DG, Benedetti F (2008). "A comprehensive review of the placebo effect: recent advances and current thought". Annual Review of Psychology. 59 (1): 565–90. doi:10.1146/annurev.psych.59.113006.095941. PMID 17550344.
- ^ Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT (February 2008). "Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration". PLOS Medicine. 5 (2): e45. doi:10.1371/journal.pmed.0050045. PMC 2253608. PMID 18303940.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Turner EH, Rosenthal R (March 2008). "Efficacy of antidepressants". BMJ. 336 (7643): 516–7. doi:10.1136/bmj.39510.531597.80. PMC 2265347. PMID 18319297.
- ^ Fountoulakis KN, Möller HJ (April 2011). "Efficacy of antidepressants: a re-analysis and re-interpretation of the Kirsch data". The International Journal of Neuropsychopharmacology. 14 (3): 405–12. doi:10.1017/S1461145710000957. PMID 20800012.
- ^ Khan A, Redding N, Brown WA (August 2008). "The persistence of the placebo response in antidepressant clinical trials". Journal of Psychiatric Research. 42 (10): 791–6. doi:10.1016/j.jpsychires.2007.10.004. PMID 18036616.
- ^ "nocebo". Mirriam-Webster Incorporated. Retrieved 22 January 2017.
- ^ Häuser W, Hansen E, Enck P (June 2012). "Nocebo phenomena in medicine: their relevance in everyday clinical practice". Deutsches Arzteblatt International. 109 (26): 459–65. doi:10.3238/arztebl.2012.0459. PMC 3401955. PMID 22833756.
- ^ "The Nocebo Effect". Priory.com. 10 February 2007. Retrieved 2009-07-08.
- ^ Shapiro AK, Chassan J, Morris LA, Frick R (1974). "Placebo induced side effects". Journal of Operational Psychiatry. 6: 43–6.
- ^ Howick, Jeremy (2020). "Unethical informed consent caused by overlooking poorly measured nocebo effects". Journal of Medical Ethics. doi:10.1136/medethics-2019-105903. PMID 32063581.
- ^ Ockene JK, Barad DH, Cochrane BB, Larson JC, Gass M, Wassertheil-Smoller S, Manson JE, Barnabei VM, Lane DS, Brzyski RG, Rosal MC, Wylie-Rosett J, Hays J (July 2005). "Symptom experience after discontinuing use of estrogen plus progestin". JAMA. 294 (2): 183–93. doi:10.1001/jama.294.2.183. PMID 16014592.
- ^ Kirsch I (1997). "Specifying non-specifics: Psychological mechanism of the placebo effect". In Harrington A (ed.). The Placebo Effect: An Interdisciplinary Exploration. Cambridge: Harvard University Press. pp. 166–86. ISBN 978-0-674-66986-4.
- ^ أ ب ت Stewart-Williams S, Podd J (March 2004). "The placebo effect: dissolving the expectancy versus conditioning debate". Psychological Bulletin. 130 (2): 324–40. doi:10.1037/0033-2909.130.2.324. PMID 14979775.
- ^ Kirsch I (1985). "Response expectancy as a determinant of experience and behavior". American Psychologist. 40 (11): 1189–1202. doi:10.1037/0003-066X.40.11.1189.
- ^ Voudouris NJ, Peck CL, Coleman G (July 1989). "Conditioned response models of placebo phenomena: further support". Pain. 38 (1): 109–16. doi:10.1016/0304-3959(89)90080-8. PMID 2780058.
- ^ Klinger R, Soost S, Flor H, Worm M (March 2007). "Classical conditioning and expectancy in placebo hypoalgesia: a randomized controlled study in patients with atopic dermatitis and persons with healthy skin". Pain. 128 (1–2): 31–9. doi:10.1016/j.pain.2006.08.025. PMID 17030095.
- ^ Colloca L, Tinazzi M, Recchia S, Le Pera D, Fiaschi A, Benedetti F, Valeriani M (October 2008). "Learning potentiates neurophysiological and behavioral placebo analgesic responses". Pain. 139 (2): 306–14. doi:10.1016/j.pain.2008.04.021. PMID 18538928.
- ^ Linde K, Witt CM, Streng A, Weidenhammer W, Wagenpfeil S, Brinkhaus B, Willich SN, Melchart D (April 2007). "The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain". Pain. 128 (3): 264–71. doi:10.1016/j.pain.2006.12.006. PMID 17257756.
- ^ Geers AL, Weiland PE, Kosbab K, Landry SJ, Helfer SG (August 2005). "Goal activation, expectations, and the placebo effect". Journal of Personality and Social Psychology. 89 (2): 143–59. doi:10.1037/0022-3514.89.2.143. PMID 16162050.
- ^ Oken BS (November 2008). "Placebo effects: clinical aspects and neurobiology". Brain. 131 (Pt 11): 2812–23. doi:10.1093/brain/awn116. PMC 2725026. PMID 18567924.
- ^ Lidstone SC, Stoessl AJ (2007). "Understanding the placebo effect: contributions from neuroimaging". Molecular Imaging and Biology. 9 (4): 176–85. doi:10.1007/s11307-007-0086-3. PMID 17334853.
- ^ أ ب Goffaux P, Redmond WJ, Rainville P, Marchand S (July 2007). "Descending analgesia--when the spine echoes what the brain expects". Pain. 130 (1–2): 137–43. doi:10.1016/j.pain.2006.11.011. PMID 17215080.
- ^ Qiu YH, Wu XY, Xu H, Sackett D (October 2009). "Neuroimaging study of placebo analgesia in humans". Neuroscience Bulletin. 25 (5): 277–82. doi:10.1007/s12264-009-0907-2. PMC 5552608. PMID 19784082.
- ^ Zubieta JK, Stohler CS (March 2009). "Neurobiological mechanisms of placebo responses". Annals of the New York Academy of Sciences. 1156 (1): 198–210. Bibcode:2009NYASA1156..198Z. doi:10.1111/j.1749-6632.2009.04424.x. PMC 3073412. PMID 19338509.
- ^ Levine JD, Gordon NC, Fields HL (September 1978). "The mechanism of placebo analgesia". Lancet. 2 (8091): 654–7. doi:10.1016/s0140-6736(78)92762-9. PMID 80579.
- ^ Faria V, Fredrikson M, Furmark T (July 2008). "Imaging the placebo response: a neurofunctional review". European Neuropsychopharmacology. 18 (7): 473–85. doi:10.1016/j.euroneuro.2008.03.002. PMID 18495442.
- ^ Diederich NJ, Goetz CG (August 2008). "The placebo treatments in neurosciences: New insights from clinical and neuroimaging studies". Neurology. 71 (9): 677–84. doi:10.1212/01.wnl.0000324635.49971.3d. PMID 18725593.
- ^ Ader R, Cohen N (1975). "Behaviorally conditioned immunosuppression". Psychosomatic Medicine. 37 (4): 333–40. doi:10.1097/00006842-197507000-00007. PMID 1162023.
- ^ أ ب Pacheco-López G, Engler H, Niemi MB, Schedlowski M (September 2006). "Expectations and associations that heal: Immunomodulatory placebo effects and its neurobiology". Brain, Behavior, and Immunity. 20 (5): 430–46. doi:10.1016/j.bbi.2006.05.003. PMID 16887325.
- ^ Colloca L, Benedetti F (July 2005). "Placebos and painkillers: is mind as real as matter?". Nature Reviews. Neuroscience. 6 (7): 545–52. doi:10.1038/nrn1705. PMID 15995725.
- ^ Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK (November 2005). "Neurobiological mechanisms of the placebo effect". The Journal of Neuroscience. 25 (45): 10390–402. doi:10.1523/JNEUROSCI.3458-05.2005. PMC 6725834. PMID 16280578.
- ^ Murray D, Stoessl AJ (December 2013). "Mechanisms and therapeutic implications of the placebo effect in neurological and psychiatric conditions". Pharmacology & Therapeutics. 140 (3): 306–18. doi:10.1016/j.pharmthera.2013.07.009. PMID 23880289.
- ^ أ ب Golomb BA, Erickson LC, Koperski S, Sack D, Enkin M, Howick J (October 2010). "What's in placebos: who knows? Analysis of randomized, controlled trials". Annals of Internal Medicine. 153 (8): 532–5. doi:10.7326/0003-4819-153-8-201010190-00010. PMID 20956710.
- ^ أ ب Hróbjartsson A, Kaptchuk TJ, Miller FG (November 2011). "Placebo effect studies are susceptible to response bias and to other types of biases". Journal of Clinical Epidemiology. 64 (11): 1223–9. doi:10.1016/j.jclinepi.2011.01.008. PMC 3146959. PMID 21524568.
- ^ Benedetti, Fabrizio (2008-10-16). Placebo Effects. Oxford University Press. ISBN 978-0-19-955912-1.
وصلات خارجية
- The Placebo Effect at the Skeptic's Dictionary
- The Placebo Effect explained on YouTube
- Interview on Placebos Richard Dawkins and Nicholas Humphrey
- Placebos: cracking the code part 1 part 2 BBC/Discovery channel program
- "The Placebo Effect: Do You Believe Your Teacher?" Ben Goldacre on The Guardian and on Wikibooks.
![The placebo effect can be produced by inert tablets, by sham surgery, and by false information, such as when electrical stimulation is turned “off” in those with Parkinson’s disease implanted brain electrodes[1]](/w/images/1/1c/Cebocap.jpg)
![The placebo effect can be produced by inert tablets, by sham surgery, and by false information, such as when electrical stimulation is turned “off” in those with Parkinson’s disease implanted brain electrodes[1]](/w/images/thumb/9/91/Parkinson_surgery.jpg/230px-Parkinson_surgery.jpg)
