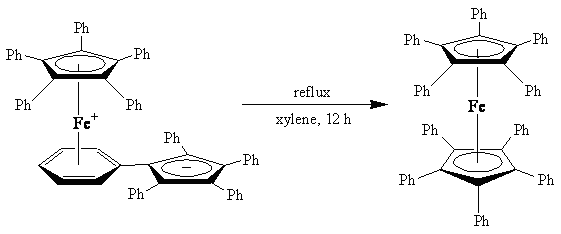
تماكب
في الكيمياء، التماكب أو التزامر أو الأزمرة (isomerization) هو تحول جزيء أو أيون متعدد الذرات أو شظية جزيئية إلى متزامر (isomer ) ببنية كيميائية مختلفة.[1] وفى بعض الجزيئيات وتحت ظروف معينة، يحدث التزامر بصورة تلقائية.[2] Enolization is an example of isomerization, as is tautomerization.[3] When the isomerization occurs intramolecularly it may be called a rearrangement reaction.
When the activation energy for the isomerization reaction is sufficiently small, both isomers will exist in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, , have been calculated, with good agreement between observed and calculated data.[4]
أمثلة وتطبيقات التزامر
Alkanes
Skeletal isomerization occurs in the cracking process, used in the petrochemical industry. As well as reducing the average chain length, straight-chain hydrocarbons are converted to branched isomers in the process, as illustrated the following reaction of n-butane to i-butane.[citation needed]
Fuels containing branched hydrocarbons are favored for internal combustion engines for their higher octane rating.[5]
Alkenes
Terminal alkenes isomerize to internal alkenes in the presence of metal catalysts. This process is employed in the Shell higher olefin process to convert alpha-olefins to internal olefins, which are subjected to olefin metathesis. In certain kinds of alkene polymerization reactions, chain walking is an isomerization process that introduces branches into growing polymers.[citation needed]
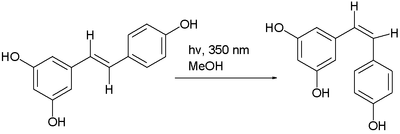
The trans isomer of resveratrol can be converted to the cis isomer in a photochemical reaction.[6]
الهامش
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "isomerization".
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "isomerization".
- ^ Antonov L (2016). Tautomerism: Concepts and Applications in Science and Technology (1st ed.). Weinheim, Germany: Wiley-VCH. ISBN 978-3-527-33995-2.
- ^ How to Compute Isomerization Energies of Organic Molecules with Quantum Chemical Methods Stefan Grimme, Marc Steinmetz, and Martin Korth J. Org. Chem.; 2007; 72(6) pp 2118 - 2126; (Article) DOI:10.1021/jo062446p
- ^ "Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2002. doi:10.1002/14356007.a13_227. ISBN 3527306730.
{{cite encyclopedia}}: Cite uses deprecated parameter|authors=(help) - ^ Resveratrol Photoisomerization: An Integrative Guided-Inquiry Experiment Elyse Bernard, Philip Britz-McKibbin, Nicholas Gernigon Vol. 84 No. 7 July 2007 Journal of Chemical Education 1159.
المصادر
- ويكيبيديا الإنجليزية.