ألومينوسيليكات
الألومينوسيليكات Aluminosilicate هي معادن مكونة من الألومنيوم، السليكون و الأكسجين، زائد countercations. وهم مكون رئيسي للكاولين و معادن طينية أخرى.
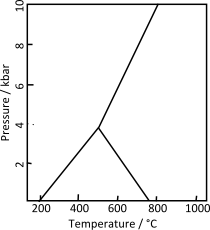
الأندلسيت والكيانيت و السيليمانيت هي معادن ألومينوسيليكات متواجدة في الطبيعة يدخل في تركيبها Al2SiO5.[2][3][4] النقطة الثلاثية لثلاث متعددات أشكال تقع عند درجة حرارة 500 °س وضغط 0.4 GPa. هذه المعادن الثلاث يشيع استخدامها كـ index minerals في الصخور المتحولة.
Hydrated aluminosilicate minerals are referred to as zeolites and are porous structures that are naturally occurring materials.
The catalyst silica-alumina is an amorphous substance which is not an aluminosilicate compound.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
ممثلون مهمّون
Feldspar is a common tectosilicate aluminosilicate mineral made of potassium, sodium, and calcium cations surrounded by a negatively charged network of silicon, aluminium and oxygen atoms.
Many aluminosilicates are synthesized by reactions of silicates, aluminates, and other compounds. They have the general formula MAlO
2)(SiO
2)
x(H
2O)
y where M+ is usually H+ and Na+. The Si/Al ratio is variable, which provides a means to tune the properties.[5] Many of these materials are porous and exhibit properties of industrial value. Naturally occurring microporous, hydrous aluminosilicate minerals are also referred to as zeolites.
زجاج الألومينوسيليكات
 مقالة مفصلة: زجاج الغوريلا
مقالة مفصلة: زجاج الغوريلا
There exist a wide variety of glass types. The characteristics of these different types depend on the chemical composition of the glass with a special focus on the oxide composition. Glasses can be categorized into many different groups, one of them including the so-called aluminosilicate glasses.
الخصائص
Aluminosilicate glasses can be formulated to tolerate temperatures up to 800 °C, temperatures substantially above those of borosilicate glasses and comparable to ceramic materials, maximum temperatures being determined by the material's transformation temperature.
Their formulations can also be matched to the thermal expansion coefficients of electrodes, e.g. molybdenum, making possible the creation, via hot-forming processes, of extremely tight gas-proof glass to metal seals.
زجاج الألومينوسيليكات الترابية القلوية
Characteristically, these glasses are free of alkali oxides and contain 15-25% Al2O3, 52-60% SiO2 and about 15% alkaline earths.[مطلوب توضيح] Very high transformation temperatures and softening points are typical features. Main fields of application are glass bulbs for halogen lamps, high-temperature thermometers and thermally and electrically highly loadable film resistors.
زجاج الألومينوسيليكات القلوية
The Al2O3 content of alkali aluminosilicate glasses is typically 10-25% and the alkali content over 10%. The high alkali content prepares the glass for ion exchange with bigger alkali ions in order to improve the surface compressive strength. Due to this particular feature, this glass type is especially suitable for the use in touch displays, solar cells cover glass and laminated safety glass. High transformation temperatures and outstanding mechanical properties, e.g. hardness and scratch behavior, are characteristic of this glass type.[6]
انظر أيضاً
- سليكات الألومنيوم
- معادن السليكات
- ألومينوسيليكات الكالسيوم
- ألومينوسيليكات الصوديوم
- زجاج غوريلا - نوع من زجاج ألومينوسيليكات.
المراجع
- ^ Whitney, D.L. (2002). "أندلسيت وكيانيت وسيليمانيت متعايشون: التشكل التسلسلي لثلاث Al2SiO5 أشكال أثناء التحول التدريجي بالقرب من النقطة الثلاثية، سڤريحصار، تركيا". American Mineralogist. 87 (4): 405–416. doi:10.2138/am-2002-0404.
- ^ "Andalusite, Handbook of Mineralogy" (PDF). Archived (PDF) from the original on 2012-05-16.
- ^ "Kyanite, Handbook of Mineralogy" (PDF). Archived (PDF) from the original on 2012-02-24.
- ^ "Sillimanite, Handbook of Mineralogy" (PDF). Archived (PDF) from the original on 2011-11-19.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ "Archived copy" (PDF). Archived (PDF) from the original on 2017-08-24. Retrieved 2017-08-24.
{{cite web}}: CS1 maint: archived copy as title (link)