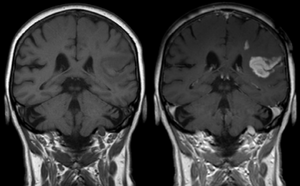
وسط تباين في التصوير بالرنين المغناطيسي

الأدوات الملوِّنة في التصوير بالرنين المغناطيسي هي مجموعة من المواد الملوِّنة التي تستخدم لتحسين الرؤية لمكونات الجسم الداخلية في التصوير بالرنين المغناطيسي(MRI)[1]. أكثر المركبات إستخداماً في تحسين التباين هي المرتكزة على "الجادلينيوم Gd". مثل هذه المواد الملونة تعمل على تقصير "وقت الاسترخاء" للأنوية في أنسجة الجسم تبعاً لإعطائها فموياً أو بالوريد. في أجهزة الرنين المغناطيسي، المقاطع من الجسم التي تتعرض لمجال مغناطيسي قوي جداً تسبب تأين -بالدرجة الأولى - لنويات الهيدروجين من جزيئات الماء في الانسجة في اتجاه المجال المغناطيسي. موجة تردد راديوية مكثفة تُسلَّط والتي بدورها تجعل المجال المغناطيسي الذي نشأ من نويّات الهيدروجين يَميل باتجاه اسلاك الاستقبال حيث ذرات الهيدروجين المُأيَّنة يُكشف عنها.التذبذب العشوائي الدوراني يطابق التردد الرنيني لنويّات الهيدروجين فتزودها "بالاسترخاء" التي تجمع المحصلة المغناطيسية لنويّات الهيدروجين وتُعيدها لوضع الاتزان بمحاذاة المجال المغناطيسي. كميّة التأين للنويّات يتم الكشف عنها باستخدام أجهزة الاستقبال التي تستخدمها لإنشاء صورة رنين مغناطيسي لكن الانحلال الثابت مع الوقت يعرف بـ وقت الاسترخاء "T1". بروتونات الماء في الانسجة المختلفة لديها قيم وقت استرخاء مختلفة، وهذا هو واحد من أهم الأسباب لوجود التباين في صورة الرنين المغناطيسي. المادة الملونة عادةً تُقصِّر وقت الاسترخاء، ولكن في بعض الحالات تزيده، قيم ال "T1" في جزيئات الماء القريبة من المادة الملونة تختل وبالتالي زيادة التباين في الصورة.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
الأنواع
گادولينيوم (Gd): مغناطيسية مسايرة
Gadolinium(III) containing MRI contrast agents (often termed simply "gado" or "gad") are the most commonly used for enhancement of vessels in MR angiography or for brain tumor enhancement associated with the degradation of the blood–brain barrier. For large vessels such as the aorta and its branches, the gadolinium(III) dose can be as low as 0.1 mmol per kg body mass. Higher concentrations are often used for finer vasculature.[2] Gd(III) chelates do not pass the intact blood–brain barrier because they are hydrophilic. Thus, these are useful in enhancing lesions and tumors where blood-brain barrier is compromised and the Gd(III) leaks out. In the rest of the body, the Gd(III) initially remains in the circulation but then distributes into the interstitial space or is eliminated by the kidneys. Gadolinium-based contrast agents appear to be safe in pregnancy.[3]
Types of gadolinium contrast agents
Gadolinium(III) contrast agents can be categorized into:
Extracellular fluid agents
- gadoterate (Dotarem, Clariscan)
- gadodiamide (Omniscan)
- gadobenate (MultiHance)
- gadopentetate (Magnevist)
- gadoteridol (ProHance)
- gadoversetamide (OptiMARK)
- gadobutrol (Gadovist [EU] / Gadavist [US])
- gadopentetic acid dimeglumine (Magnetol)
Blood pool agents
- Albumin-binding gadolinium complexes
- gadofosveset (Ablavar, formerly Vasovist)
- gadocoletic acid
- Polymeric gadolinium complexes
Hepatobiliary (liver) agents
- gadoxetic acid (Primovist [EU] / Eovist [US]) is used as a hepatobiliary agent as 50% is taken up and excreted by the liver and 50% by the kidneys.
Gadolinium-containing contrast agents approved for human use
Presently, nine different types of gadolinium-containing contrast agents are available in different territories. In European countries, Gd chelated contrast agents approved by the European Medicines Agency (EMA) include:[4]
- gadoterate (Dotarem, Clariscan)
- gadodiamide (Omniscan)
- gadobenate (MultiHance)
- gadopentetate (Magnevist, Magnegita, Gado-MRT ratiopharm)
- gadoteridol (ProHance)
- gadoversetamide (OptiMARK)
- gadoxetate (Primovist)
- gadobutrol (Gadovist)
In the United States of America, Gd chelated contrast agents approved by the U.S. Food and Drug Administration (FDA) include:[5]
- gadoterate (Dotarem)
- gadodiamide (Omniscan)
- gadobenate (MultiHance)
- gadopentetate (Magnevist)
- gadoteridol (ProHance)
- gadofosveset (Ablavar, formerly Vasovist)
- gadoversetamide (OptiMARK)
- gadoxetate (Eovist)
- gadobutrol (Gadavist)
Safety of gadolinium contrast agents
Gadolinium-based MRI contrast agents have not proved safer than the iodinated hydrophilic radiocontrast agents used in X-ray radiography or computed tomography as they are nephrotoxic and neurotoxic. Because gadolinium-based contrast agents pass the blood–brain barrier and of each bolus dose at least 1% of the gadolinium is retained and assumed to be in its free toxic state[بحاجة لمصدر] ; these products need further study. Anaphylactoid reactions are rare, occurring in approx. 0.03–0.1%.[6]
As a free solubized aqueous ion, gadolinium (III) is somewhat toxic, but was generally regarded as safe when administered as a chelated compound. In animals the free Gd (III) ion exhibits a 100–200 mg/kg 50% lethal dose, but the LD50 is increased by a factor of 100 when Gd (III) is chelated, so that its toxicity becomes comparable to iodinated X-ray contrast compounds.[7] The chelating carrier molecule for Gd for MRI contrast use can be classified by whether they are macro-cyclic or have linear geometry and whether they are ionic or not. Cyclical ionic Gd(III) compounds are considered the least likely to release the Gd(III) ion, and hence the safest.[8] However, the use of some Gd(III) chelates in persons with renal disease was linked to a rare but severe complication, nephrogenic fibrosing dermopathy,[9] also known as nephrogenic systemic fibrosis (NSF).[9][10][11] This systemic disease resembles scleromyxedema and to some extent scleroderma. It may occur months after contrast has been injected.[12] Patients with poorer renal function are more at risk for NSF, with dialysis patients being more at risk than patients with renal insufficiency.[13][14] After several years of controversy during which up to 100 Danish patients have been gadolinium poisoned (and some died) after use of the contrast agent Omniscan, it was admitted by the Norwegian medical company Nycomed that they were aware of some dangers of using gadolinium-based agents for their product.[15] At present, NSF has been linked to the use of four gadolinium-containing MRI contrast agents. The World Health Organization issued a restriction on use of several gadolinium contrast agents in November 2009 stating that "High-risk gadolinium-containing contrast agents (Optimark, Omniscan, Magnevist, Magnegita and Gado-MRT ratiopharm) are contraindicated in patients with severe kidney problems, in patients who are scheduled for or have recently received a liver transplant, and in newborn babies up to four weeks of age."[16]
Gadolinium has been found to remain in the body after multiple MRIs, even after a prolonged period of time. Although gadolinium contrast agents have not been found to be harmful to the body, it is unknown whether these deposits can lead to adverse health effects. The FDA has asked doctors to limit the use of Gadolinium contrast agents to times when necessary information is made available through its use.[17]
Continuing evidence of the retention of gadolinium in brain and other tissues following exposure to gadolinium containing contrast media, has led to a safety review by the European Medicines Agency (EMA and The Committee for Medicinal Products for Human Use (CHMP)). Although not directly linked to adverse health effects in patients with normal kidney function, the possible risk of using intravenous linear chelated media, in which the gadolinium is shown to have a lower binding affinity, has led to a change in the market authorisation for all linear chelated gadolinium-based media.
With both Gadopentetic acid (gadopentetate dimegulumine (Magnevist)) and Gadodiamide (Omniscan) the risk was considered to outweigh the benefits and, as a result, the EMA recommended the licence for intravenous gadopentetic acid and Gadodiamide be suspended. [18]
Following a legally binding decision issued by The European Commission and applicable in all EU Member States (Commission decision date: 23/11/2017), Intravenous Magnevist and Omniscan is now no longer authorised for use in Europe. Magnavist is, however, still authorised in Europe as an intra-articular MR contrast medium. The authorised indication for the use of linear chelated media gadobenic acid (also known as gadobenate dimeglumine; MultiHance) and gadoxetic acid (Primovist) has been limited to delayed phase liver imaging only. [19]
In the United States of America, the research has led the FDA (Food and Drug Administration) to revise its class warnings for all gadolinium-based contrast media. It is advised that the use of gadolinium-based media is based on careful consideration of the retention characteristics of the preferred medium. Extra care being taken in patients requiring multiple lifetime doses, pregnant and paediatric patients, and patients with inflammatory conditions. Minimizing repeated GBCA imaging studies when possible, particularly closely spaced MRI studies. However, do not avoid or defer necessary GBCA MRI scans. [20]
أكسيد الحديد
Two types of iron oxide contrast agents exist: superparamagnetic iron oxide (SPIO) and ultrasmall superparamagnetic iron oxide (USPIO). These contrast agents consist of suspended colloids of iron oxide nanoparticles and when injected during imaging reduce the T2 signals of absorbing tissues. SPIO and USPIO contrast agents have been used successfully in some instances for liver tumor enhancement.[21]
- Feridex I.V. (also known as Endorem and ferumoxides). This product was discontinued by AMAG Pharma in November 2008.[22]
- Resovist (also known as Cliavist). This was approved for the European market in 2001, but production was abandoned in 2009.[23]
- Sinerem (also known as Combidex). Guerbet withdrew the marketing authorization application for this product in 2007.[24]
- Lumirem (also known as Gastromark). Gastromark was approved by the FDA in 1996[25] and was discontinued by its manufacturer in 2012.[26][27]
- Clariscan (also known as PEG-fero, Feruglose, and NC100150). This iron based contrast agent was never commercially launched and its development was discontinued in early 2000s due to safety concerns.[28] In 2017 GE Healthcare launched a macrocyclic extracellular gadolinium based contrast agent containing gadoteric acid as gadoterate meglumine under the trade name Clariscan.[29]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Iron Platinum: Superparamagnetic
Superparamagnetic iron–platinum particles (SIPPs) have been reported and had significantly better T2 relaxivities compared with the more common iron oxide nanoparticles. SIPPs were also encapsulated with phospholipids to create multifunctional SIPP stealth immunomicelles that specifically targeted human prostate cancer cells.[30] These are, however, investigational agents which have not yet been tried in humans. In a recent study, multifunctional SIPP micelles were synthesized and conjugated to a monoclonal antibody against prostate-specific membrane antigen.[30] The complex specifically targeted human prostate cancer cells in vitro, and these results suggest that SIPPs may have a role in the future as tumor-specific contrast agents.
Manganese: Paramagnetic
Unlike the other well-studied iron oxide-based nanoparticles, research on Mn-based nanoparticles is at a relatively early stage.[31] Manganese chelates such as Mn-DPDP enhance the T1 signal and have been used for the detection of liver lesions. The chelate dissociates in vivo into manganese and DPDP where the former is absorbed intra-cellularly and excreted in bile, while the latter is eliminated via the renal filtration.[32]
Manganese ions (Mn2+) are often used as a contrast agent in animal studies, usually referred to as MEMRI (Manganese Enhanced MRI).[33] Due to the ability of Mn2+ to enter cells through Calcium Ca2+ channels Mn2+ can e.g. be used for functional brain imaging.[34]
Oral administration of contrast agents
A wide variety of oral contrast agents can enhance images of the gastrointestinal tract. They include gadolinium and manganese chelates, or iron salts for T1 signal enhancement. SPIO, barium sulfate, air and clay have been used to lower T2 signal. Natural products with high manganese concentration such as blueberry and green tea can also be used for T1 increasing contrast enhancement.[35]
Perflubron, a type of perfluorocarbon, has been used as a gastrointestinal MRI contrast agent for pediatric imaging.[36] This contrast agent works by reducing the number of hydrogen ions in a body cavity, thus causing it to appear dark in the images.
Protein-based MRI contrast agents
Newer research suggests the possibility of protein based contrast agents, based on the abilities of some amino acids to bind with gadolinium.[37][38][39][40]
انظر أيضاً
مراجع
- ^ "MR Contrast Agents". 2014. Archived 2017-07-31 at the Wayback Machine
- ^ Lentschig, MG; Reimer, P; Rausch-Lentschig, UL; Allkemper, T; Oelerich, M; Laub, G (1998). "Breath-hold gadolinium-enhanced MR angiography of the major vessels at 1.0 T: Dose-response findings and angiographic correlation". Radiology. 208 (2): 353–7. doi:10.1148/radiology.208.2.9680558. PMID 9680558.
- ^ Garcia-Bournissen F, Shrim A, Koren G (2006). "Safety of gadolinium during pregnancy". Can Fam Physician. 52: 309–10. PMC 1479713. PMID 16572573.
- ^ "EMA recommendations on Gadolinium-containing contrast agents". ema.europa.eu. Retrieved 2018-07-12.
- ^ "Information on Gadolinium-Containing Contrast Agents". Fda.gov. Retrieved 2018-07-12.
- ^ Murphy KJ, Brunberg JA, Cohan RH; Brunberg; Cohan (1 October 1996). "Adverse reactions to gadolinium contrast media: A review of 36 cases". AJR Am J Roentgenol. 167 (4): 847–9. doi:10.2214/ajr.167.4.8819369. PMID 8819369.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Penfield, Jeffrey G; Reilly, Robert F (2007). "What nephrologists need to know about gadolinium". Nature Clinical Practice Nephrology. 3 (12): 654–68. doi:10.1038/ncpneph0660. PMID 18033225.
- ^ "Questions and Answers" (PDF). International Society for Magnetic Resonance in Medicine.
- ^ أ ب Grobner, T. (2005). "Gadolinium - a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?". Nephrology Dialysis Transplantation. 21 (4): 1104–8. doi:10.1093/ndt/gfk062. PMID 16431890.
- ^ Marckmann, P.; Skov, L; Rossen, K; Dupont, A; Damholt, MB; Heaf, JG; Thomsen, HS (2006). "Nephrogenic Systemic Fibrosis: Suspected Causative Role of Gadodiamide Used for Contrast-Enhanced Magnetic Resonance Imaging". Journal of the American Society of Nephrology. 17 (9): 2359–62. doi:10.1681/ASN.2006060601. PMID 16885403.
- ^ Centers for Disease Control and Prevention (CDC) (2007). "Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents--St. Louis, Missouri, 2002-2006". MMWR. Morbidity and Mortality Weekly Report. 56 (7): 137–41. PMID 17318112.
- ^ Thomsen, H.S.; Morcos, S.K.; Dawson, P. (2006). "Is there a causal relation between the administration of gadolinium based contrast media and the development of nephrogenic systemic fibrosis (NSF)?". Clinical Radiology. 61 (11): 905–6. doi:10.1016/j.crad.2006.09.003. PMID 17018301.
- ^ Kanal, E.; Barkovich, A. J.; Bell, C.; Borgstede, J. P.; Bradley, W. G.; Froelich, J. W.; Gilk, T.; Gimbel, J. R.; et al. (2007). "ACR Guidance Document for Safe MR Practices: 2007". American Journal of Roentgenology. 188 (6): 1447–74. doi:10.2214/AJR.06.1616. PMID 17515363.
- ^ "Gadolinium and NSF What is fact and what is theory?". 2008.
- ^ "Nyhedsavisen: Medicinalfirma fortiede at stof var farligt". Retrieved 2010-11-05.
- ^ "Pharmaceuticals: Restrictions in Use and Availability" (PDF). World Health Organization. 2010. p. 14.
- ^ http://www.fda.gov/Drugs/DrugSafety/ucm455386.htm
- ^ http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Gadolinium-containing_contrast_agents/human_referral_prac_000056.jsp&mid=WC0b01ac05805c516f
- ^ https://www.gov.uk/drug-safety-update/gadolinium-containing-contrast-agents-removal-of-omniscan-and-iv-magnevist-restrictions-to-the-use-of-other-linear-agents
- ^ https://www.fda.gov/downloads/Drugs/DrugSafety/UCM589442.pdf
- ^ Nakamura, Hiroshi; Ito, Naoki; Kotake, Fumio; Mizokami, Yuji; Matsuoka, Takeshi (2000). "Tumor-detecting capacity and clinical usefulness of SPIO-MRI in patients with hepatocellular carcinoma". Journal of Gastroenterology. 35 (11): 849–55. doi:10.1007/s005350070022. PMID 11085494.
- ^ "Feridex - Products - AMAG Pharmaceuticals". Amagpharma.com. Archived from the original on 2012-06-15. Retrieved 2012-06-20.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Softways. "Magnetic Resonance TIP - MRI Database : Resovist". Mr-tip.com. Retrieved 2012-06-20.
- ^ "AMAG Pharmaceuticals, Inc. Announces Update on Sinerem(TM) in Europe. - Free Online Library". Thefreelibrary.com. 2007-12-13. Retrieved 2012-06-20.
- ^ "Newly Approved Drug Therapies (105) GastroMARK, Advanced Magnetics". CenterWatch. Retrieved 2012-06-20.
- ^ "AMAG Form 10-K For the Fiscal Year Ended December 31, 2013". SEC Edgar.
- ^ "NDA 020410 for GastroMark". FDA. Retrieved 12 February 2017.
- ^ Wang, Yi-Xiang J. (2011). "Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application". Quantitative Imaging in Medicine and Surgery. 1 (1): 35–40. doi:10.3978/j.issn.2223-4292.2011.08.03. PMC 3496483. PMID 23256052.
- ^ http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1487912585095.pdf
- ^ أ ب Taylor, Robert M.; Huber, Dale L.; Monson, Todd C.; Ali, Abdul-Mehdi S.; Bisoffi, Marco; Sillerud, Laurel O. (2011). "Multifunctional iron platinum stealth immunomicelles: Targeted detection of human prostate cancer cells using both fluorescence and magnetic resonance imaging". Journal of Nanoparticle Research. 13 (10): 4717–4729. doi:10.1007/s11051-011-0439-3. PMC 3223933. PMID 22121333.
- ^ Zhen, Zipeng; Xie, J (2012). "Development of Manganese-Based Nanoparticles as Contrast Probes for Magnetic Resonance Imaging". Theranostics. 2 (1): 45–54. doi:10.7150/thno.3448. PMC 3263515. PMID 22272218.
- ^ Harisinghani, Mukesh G.; Jhaveri, Kartik S.; Weissleder, Ralph; Schima, Wolfgang; Saini, Sanjay; Hahn, Peter F.; Mueller, Peter R. (2001). "MRI Contrast Agents for Evaluating Focal Hepatic Lesions". Clinical Radiology. 56 (9): 714–25. doi:10.1053/crad.2001.0764. PMID 11585393.
- ^ Koretsky, Alan P.; Silva, Afonso C. (2004). "Manganese-enhanced magnetic resonance imaging (MEMRI)". NMR in Biomedicine. 17 (8): 527–31. doi:10.1002/nbm.940. PMC 3285478. PMID 15617051.
- ^ Lin, Yi-Jen; Koretsky, Alan P. (1997). "Manganese ion enhances T1-weighted MRI during brain activation: An approach to direct imaging of brain function". Magnetic Resonance in Medicine. 38 (3): 378–88. doi:10.1002/mrm.1910380305. PMID 9339438.
- ^ Computed Body Tomography with MRI Correlation. ISBN 978-0-7817-4526-0.[صفحة مطلوبة]
- ^ Bisset, G. S.; Emery, K. H.; Meza, M. P.; Rollins, N. K.; Don, S.; Shorr, J. S. (1996). "Perflubron as a gastrointestinal MR imaging contrast agent in the pediatric population". Pediatric Radiology. 26 (6): 409–15. doi:10.1007/BF01387316. PMID 8657479.
- ^ Xue, Shenghui; Qiao, Jingjuan; Pu, Fan; Cameron, Mathew; Yang, Jenny J. (2013). "Design of a novel class of protein-based magnetic resonance imaging contrast agents for the molecular imaging of cancer biomarkers". Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 5 (2): 163–79. doi:10.1002/wnan.1205. PMC 4011496. PMID 23335551.
- ^ Li, Shunyi; Jiang, Jie; Zou, Jin; Qiao, Jingjuan; Xue, Shenghui; Wei, Lixia; Long, Robert; Wang, Liya; et al. (2012). "PEGylation of protein-based MRI contrast agents improves relaxivities and biocompatibilities". Journal of Inorganic Biochemistry. 107 (1): 111–8. doi:10.1016/j.jinorgbio.2011.11.004. PMC 3273044. PMID 22178673.
- ^ Xue, Shenghui; Qiao, Jingjuan; Hubbard, Kendra; White, Natalie; Wei, Lixia; Li, Shunyi; Liu, Zhi-Reb; Yang, Jenny J; Yang, J. J. (2014). "Design of ProCAs (Protein-Based Gd3+ MRI Contrast Agents) with High Dose Efficiency and Capability for Molecular Imaging of Cancer Biomarkers". Medicinal Research Reviews. 34 (5): 1070–99. doi:10.1002/med.21313. PMID 24615853.
- ^ Qiao, Jingjuan; Xue, Shenghui; Pu, Fan; White, Natalie; Liu, Zhi-Ren; Yang, Jenny J. (2014). "Molecular imaging of EGFR/HER2 cancer biomarkers by protein MRI contrast agents". J Biol Inorg Chem. 19 (2): 259–70. doi:10.1007/s00775-013-1076-3. PMC 3931309. PMID 24366655.