خلية جذعية جنينية
| إيلان ساهم بشكل رئيسي في تحرير هذا المقال
|
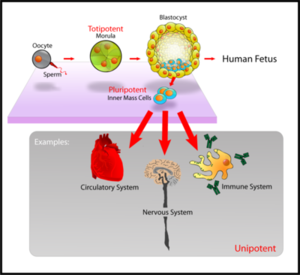
الخلية الجذعية الجنينية Embryonic stem cells (ES cells) هي خلية جذعية قابلة للانقسام تنشأ من inner cell mass of the blastocyst, an early-stage embryo.[1] Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells.
خطوط الخلايا الجذعية الجنينية (embryonic stem cell lines) عبارة عن تجمعات أو مزارع خلوية تشتق من النسيج الأصيلي الخارجي epiblast tissue من كتلة الخلايا الداخلية inner cell mass للكيسة الأرومية. الكيسة الأرومية تكون عادة مرحلة مبكرة من التطور الجنيني بعمر حوالي 4-5 أيام في الإنسان وتتألف من 50-150 خلية. في هذه الحالة تكون الخلايا الجذعية متعددة الخيارات وتعطي خلال نموها منتجات الطبقات الجنينية الثلاث: الطبقة الجنينية الخارجية ectoderm والوسطى mesoderm والداخلية endoderm. هذا يعني أن الخلايا الجذعية في هذه الحالة قادرة على التنامي نحو أكثر من 200 نمط خلوي موجود في الجسم البالغ، وكل ما هو مطلوب هو إعطاء التنبيه المناسب لكل نمط خلوي نوعي. وهذه الخلايا لا تشارك في الأغشية الخارج-جنينية أو ضمن تركيب المشيمة placenta.
في الزجاج، وإذا لم يعطى أي منبه تمايزي محدد، تستمر الخلايا الجذعية بالانقسام وكل خلية ابنة daughter cell ستبقى متعددة الخيارات. وتعدد الخيارات هذا للخلايا الجذعية الجنينية تم بحثه واستقصائه سواء ضمن الزجاج أو ضمن الحيوية، لذلك يمكن تصنيفها فعلا على أنها خلايا جذعية فعلا.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
الخصائص
تعتبر الخلايا الجذعية الجنينية بسبب قدرتها الانقسامية اللامحدودة وتعدد خياراتها، مصدرا كامنا للعديد من الأفكار في مجال الطب الاستبدالي واستبدال الأعضاء بعد أذيتها أو مرضها.
تطور الأبحاث حول الخلايا الجذعية
Techniques and Conditions for Embryonic Stem Cell Derivation and Culture
Embryonic stem cells are derived from the inner cell mass of the early embryo, which are harvested from the donor mother animal. Martin Evans and Matthew Kaufman reported a technique that delays embryo implantation, allowing the inner cell mass to increase. This process includes removing the donor mother’s ovaries and dosing her with progesterone, changing the hormone environment, which causes the embryos to remain free in the uterus. After 4–6 days of this intrauterine culture, the embryos are harvested and grown in in vitro culture until the inner cell mass forms “egg cylinder-like structures,” which are dissociated into single cells, and plated on fibroblasts treated with mitomycin-c (to prevent fibroblast mitosis). Clonal cell lines are created by growing up a single cell. Evans and Kaufman showed that the cells grown out from these cultures could form teratomas and embryoid bodies, and differentiate in vitro, which all indicate the cells are pluripotent.[2]
Gail Martin derived and cultured her ES cells differently. She removed the embryos from the donor mother at approximately 76 hours after copulation and cultured them overnight in media containing serum. The following day, she removed the inner cell mass from the late blastocyst using microsurgery. The extracted inner cell mass was cultured on fibroblasts treated with mitomycin-c in media that containing serum and was conditioned by EC cells. After approximately one week, colonies of cells grew out. These cells grew in culture and demonstrated pluripotent characteristics, as demonstrated by the ability to form teratomas, differentiate in vitro, and form embryoid bodies. Martin referred to these cells as ES cells.[3]
It is now known that the feeder cells provide leukemic inhibitory factor (LIF) and serum provides bone morphogenetic proteins (BMPs) that are necessary to prevent ES cells from differentiating.[4][5] These factors are extremely important for the efficiency of deriving ES cells. Furthermore, it has been demonstrated that different mouse strains have different efficiencies for isolating ES cells.[6] Current uses for mouse ES cells include the generation of transgenic mice, including knockout mice. For human treatment, there is a need for patient specific pluripotent cells. Generation of human ES cells is more difficult and faces ethical issues. So, in addition to human ES cell research, many groups are focused on the generation of induced pluripotent stem cells (iPS cells).[7]
Contamination by reagents used in cell culture
The online edition of Nature Medicine published a study on January 24, 2005 which stated that the human embryonic stem cells available for federally funded research are contaminated with non-human molecules from the culture medium used to grow the cells.[8] It is a common technique to use mouse cells and other animal cells to maintain the pluripotency of actively dividing stem cells. The problem was discovered when non-human sialic acid in the growth media was found to compromise the potential uses of the embryonic stem cells in humans, according to scientists at the University of California, San Diego.[9]
However, a study published in the online edition of Lancet Medical Journal on March 8, 2005 detailed information about a new stem cell line which was derived from human embryos under completely cell- and serum-free conditions. After more than 6 months of undifferentiated proliferation, these cells demonstrated the potential to form derivatives of all three embryonic germ layers both in vitro and in teratomas. These properties were also successfully maintained (for more than 30 passages) with the established stem cell lines.[10]
Reducing donor-host rejection
There is also ongoing research to reduce the potential for rejection of the differentiated cells derived from ES cells once researchers are capable of creating an approved therapy from ES cell research. One of the possibilities to prevent rejection is by creating embryonic stem cells that are genetically identical to the patient via therapeutic cloning.
An alternative solution for rejection by the patient to therapies derived from non-cloned ES cells is to derive many well-characterized ES cell lines from different genetic backgrounds and use the cell line that is most similar to the patient; treatment can then be tailored to the patient, minimizing the risk of rejection.
First Embryonic Stem Cell Trial Approved by the FDA
On January 23, 2009, Phase I clinical trials for transplantation of a human-ES-derived cell population into spinal cord-injured individuals received approval from the U.S. Food and Drug Administration (FDA), marking it the world's first human ES cell human trial.[11] The study leading to this scientific advancement was conducted by Hans Keirstead and colleagues at the University of California, Irvine and supported by Geron Corporation of Menlo Park, CA. The results of this experiment suggested an improvement in locomotor recovery in spinal cord-injured rats after a 7-day delayed transplantation of human ES cells that were pushed towards an oligodendrocytic lineage.[12] In the proposed phase I clinical study, about eight to ten paraplegics who have had their injuries no longer than two weeks before the trial begins, will be selected, since the cells must be injected before scar tissue is able to form. However, the researchers are emphasizing that the injections are not expected to fully cure the patients and restore all mobility. Based on the results of the rodent trials, researchers say restoration of myelin sheathes, and an increase in mobility is probable. This first trial is mainly testing the safety of these procedures and if everything goes well, it could lead to future studies that involve people with more severe disabilities.[13] Unfortunately the trial is on hold since August 2009 due to concerns made by the FDA regarding a small number of microscopic cysts found in several treated rat models. If all goes well with Gerons follow-up experiments the clinical trial should resume by the end of 2010.[14]
Potential method for new cell line derivation
On August 23, 2006, the online edition of Nature scientific journal published a letter by Dr. Robert Lanza (medical director of Advanced Cell Technology in Worcester, MA) stating that his team had found a way to extract embryonic stem cells without destroying the actual embryo.[15] This technical achievement would potentially enable scientists to work with new lines of embryonic stem cells derived using public funding in the USA, where federal funding was at the time limited to research using embryonic stem cell lines derived prior to August 2001. In March, 2009, the limitation was lifted.[16]
Recently, it was shown that pluripotent stem cells highly similar to embryonic stem cells can be generated by the delivery of three genes (Oct4, Sox2, and Klf4) to differentiated cells.[17] The delivery of these genes "reprograms" differentiated cells into pluripotent stem cells, allowing for the generation of pluripotent stem cells without the embryo. Because ethical concerns regarding embryonic stem cells typically are about their derivation from terminated embryos, it is believed that reprogramming to these "induced pluripotent stem cells" (iPS cells) may be less controversial. Both human and mouse cells can be reprogrammed by this methodology, generating both human pluripotent stem cells and mouse pluripotent stem cells without an embryo.[18]
This may enable the generation of patient specific ES cell lines that could potentially be used for cell replacement therapies. In addition, this will allow the generation of ES cell lines from patients with a variety of genetic diseases and will provide invaluable models to study those diseases.
However, as a first indication that the induced pluripotent stem cell (iPS) cell technology can in rapid succession lead to new cures, it was used by a research team headed by Rudolf Jaenisch of the Whitehead Institute for Biomedical Research in Cambridge, مساتشوستس, to cure mice of sickle cell anemia, as reported by Science journal's online edition on December 6, 2007.[19]
On January 16, 2008, a California based company, Stemagen, announced that they had created the first mature cloned human embryos from single skin cells taken from adults. These embryos can be harvested for patient matching embryonic stem cells.[20]
Use of human embryonic stem cells as models for human genetic disorders
In recent years there have been several reports regarding the potential use of human embryonic stem cells as models for human genetic diseases. This issue is especially important due to the species-specific nature of many genetic disorders. The relative inaccessibility of human primary tissue for research is another major hindrance. Several new studies have started to address this issue. This has been done either by genetically manipulating the cells, or more recently by deriving diseased cell lines identified by prenatal genetic diagnosis (PGD). This approach may very well prove invaluable at studying disorders such as Fragile-X syndrome, Cystic fibrosis, and other genetic maladies that have no reliable model system.
Yury Verlinsky (Sept, 1, 1943 - July 16, 2009), a Russian-American medical researcher who specialized in embryo and cellular genetics (genetic cytology), developed prenatal diagnosis testing methods to determine genetic and chromosomal disorders a month and a half earlier than standard amniocentesis. The techniques are now used by many pregnant women and prospective parents, especially those couples with a history of genetic abnormalities or where the woman is over the age of 35, when the risk of genetically-related disorders is higher. In addition, by allowing parents to select an embryo without genetic disorders, they have the potential of saving the lives of siblings that already had similar disorders and diseases using cells from the disease free offspring.[21]
الجانب الأخلاقي والديني والإنساني
أثارت تجارب الخلايا الجذعية من الأجنة البشرية جدلا أخلاقيا واسعا خصوصا من قبل الجماعات المناهضة للإجهاض، والمحافظين في الغرب.
وهناك بعض الاختلافات الدينية، فالدين الإسلامي واليهودية يؤيدان بحوث الخلايا الجذعية من الأجنة البشرية قبل نفخ الروح في الجنين، ولا تجوز هذه البحوث بعد 121 يوما في المذهب السني، وبعد ثلاثة أشهر في المذهب الشيعي، وبعد 41 يوما في الديانة اليهودية، أما المسيحية فمعظم طوائفها تعارض إجراء بحوث على الخلايا الجذعية من الجنين البشري من اليوم الأول للحمل.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
أما بالنسبة للبحوث من مصادر أخرى غير الأجنة البشرية
كالحصول على الخلايا الجذعية من دم الحبل السري أو المشيمة أو نخاع العظام فلا تختلف الأديان السماوية الثلاث حول جواز تلك البحوث.
مرحلة البلاستولة
هي نتيجة لعدة انقسامات للخلية الكاملة الفعالية وهي الخلية القادرة على تكوين إنسان كامل بمختلف أعضائه، وتتكون الخلية الكاملة الفعالية عندما يلقح الحيوان المنوي البويضة.
وتتكون البلاستولة من طبقة خارجية من الخلايا مسؤولة عن تكوين المشيمة والأنسجة الداعمة الأخرى التي يحتاج إليها الجنين أثناء عم خلية جذعية متعلقة باللحمة المتوسطة.
انظر أيضا
- Embryonic Stem Cell Research Oversight (ESCRO) Committees
- Embryoid body
- نقد الخلايا الجذعية
- Fetal tissue implant
المصادر
- ^ Thomson; et al. (1998). "Blastocysts Embryonic Stem Cell Lines Derived from Human". Science. 282 (1145): 1145–1147. doi:10.1126/science.282.5391.1145.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help) - ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةEvans M, Kaufman M 1981 154–6 - ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةMartin G 1981 7634–8 - ^ Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D (1988). "Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides". Nature. 336 (6200): 688–690. doi:10.1038/336688a0. PMID 3143917.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM (1988). "Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells". Nature. 336 (6200): 684–687. doi:10.1038/336684a0. PMID 3143916.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ledermann B, Bürki K (1991). "Establishment of a germ-line competent C57BL/6 embryonic stem cell line". Exp Cell Res. 197 (2): 254–258. doi:10.1016/0014-4827(91)90430-3. PMID 1959560.
- ^ Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. (2007). "Induction of pluripotent stem cells from adult human fibroblasts by defined factors". Cell. 131 (5): 861–872. doi:10.1016/j.cell.2007.11.019. PMID 18035408.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ebert, Jessica (24 January 2005). "Human stem cells trigger immune attack". News from "Nature". London: Nature Publishing Group. doi:10.1038/news050124-1. Retrieved 2009-02-27.
- ^ Martin MJ, Muotri A, Gage F, Varki A (2005). "Human embryonic stem cells express an immunogenic nonhuman sialic acid". Nat. Med. 11 (2): 228–32. doi:10.1038/nm1181. PMID 15685172.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Klimanskaya I, Chung Y, Meisner L, Johnson J, West MD, Lanza R (2005). "Human embryonic stem cells derived without feeder cells". Lancet. 365 (9471): 1636–41. doi:10.1016/S0140-6736(05)66473-2. PMID 15885296.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "FDA approves human embryonic stem cell study - CNN.com". January 23, 2009. Retrieved May 1, 2010.
- ^ Keirstead HS, Nistor G, Bernal G; et al. (2005). "Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury". J. Neurosci. 25 (19): 4694–705. doi:10.1523/JNEUROSCI.0311-05.2005. PMID 15888645.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Steven Reinberg FDA OKs 1st Embryonic Stem Cell Trial
- ^ Geron comments on FDA hold on spinal cord injury trial http://www.geron.com/media/pressview.aspx?id=1188
- ^ Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. (2006). "Human embryonic stem cell lines derived from single blastomeres". Nature. 444 (7118): 481–5. doi:10.1038/nature05142. PMID 16929302.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ US scientists relieved as Obama lifts ban on stem cell research, The Guardian, 10 March 2009
- ^ "Human stem cells may be produced without embryos 'within months'". Zangani. 2007-07-17.
- ^ "Embryonic stem cells made without embryos". Reuters. 2007-11-21.
- ^ Rick Weiss (2007-12-07). "Scientists Cure Mice Of Sickle Cell Using Stem Cell Technique: New Approach Is From Skin, Not Embryos". Washington Post. pp. A02.
- ^ Helen Briggs (2008-01-17). "US team makes embryo clone of men". BBC. pp. A01.
- ^ "Dr. Yury Verlinsky, 1943-2009: Expert in reproductive technology" Chicago Tribune, July 20, 2009
وصلات خارجية
- Understanding Stem Cells: A View of the Science and Issues from the National Academies
- National Institutes of Health
- The Center for Bioethics & Human Dignity
- Information & Alternatives to Embryonic Stem Cell Research
المصادر: الخلايا الجذعية الجنينية Embryonic stem cell | الخلايا الجذعية البالغة Adult stem cell | الخلايا الجذعية السرطانية Cancer stem cell
مقالات متعلقة: معالجات الخلايا الجذعية Stem cell treatments | الخلاف حول الخلايا الجذعية Stem cell controversy | Stem cell line | خلية سليفة Progenitor cell | تمايز خلوي Cell differentiation