أصل الحياة

. حسب المقال فإن هذا يعتبر دليلاً على إحدى أشكال الحياة المبكرة على الأرض.]]
أصل الحياة Abiogenesis (من a-‘غير’ + اليونانية bios ‘حياة’ + genesis 'أصل') أو نشوء الحياة على الأرض[2] هي العملية الطبيعية لنشوء الحياة من مادة غير حية مثل المركبات العضوية البسيطة]]. The prevailing scientific hypothesis is that the transition from non-living to living entities was not a single event, but an evolutionary process of increasing complexity that involved the formation of a habitable planet, the prebiotic synthesis of organic molecules, molecular self-replication, self-assembly, autocatalysis, and the emergence of cell membranes. Many proposals have been made for different stages of the process.
The study of abiogenesis aims to determine how pre-life chemical reactions gave rise to life under conditions strikingly different from those on Earth today. It primarily uses tools from biology and chemistry, with more recent approaches attempting a synthesis of many sciences. Life functions through the specialized chemistry of carbon and water, and builds largely upon four key families of chemicals: lipids for cell membranes, carbohydrates such as sugars, amino acids for protein metabolism, and nucleic acids DNA and RNA for the mechanisms of heredity. Any successful theory of abiogenesis must explain the origins and interactions of these classes of molecules. Many approaches to abiogenesis investigate how self-replicating molecules, or their components, came into existence. Researchers generally think that current life descends from an RNA world, although other self-replicating molecules may have preceded RNA.
The classic 1952 Miller–Urey experiment demonstrated that most amino acids, the chemical constituents of proteins, can be synthesized from inorganic compounds under conditions intended to replicate those of the early Earth. External sources of energy may have triggered these reactions, including lightning, radiation, atmospheric entries of micro-meteorites and implosion of bubbles in sea and ocean waves. Other approaches ("metabolism-first" hypotheses) focus on understanding how catalysis in chemical systems on the early Earth might have provided the precursor molecules necessary for self-replication.
A genomics approach has sought to characterise the last universal common ancestor (LUCA) of modern organisms by identifying the genes shared by Archaea and Bacteria, members of the two major branches of life (where the Eukaryotes belong to the archaean branch in the two-domain system). 355 genes appear to be common to all life; their nature implies that the LUCA was anaerobic with the Wood–Ljungdahl pathway, deriving energy by chemiosmosis, and maintaining its hereditary material with DNA, the genetic code, and ribosomes. Although the LUCA lived over 4 billion years ago (Gya), researchers do not believe it was the first form of life. Earlier cells might have had a leaky membrane and been powered by a naturally-occurring proton gradient near a deep-sea white smoker hydrothermal vent.
Earth remains the only place in the universe known to harbor life, and fossil evidence from the Earth informs most studies of abiogenesis. The Earth was formed 4.54 Gya; the earliest undisputed evidence of life on Earth dates from at least 3.5 Gya. Fossil micro-organisms appear to have lived within hydrothermal vent precipitates dated 3.77 to 4.28 Gya from Quebec, soon after ocean formation 4.4 Gya during the Hadean.
لنظرة عامة حول نشأة الحياة على الأرض انظر: الخط الزمني للحياة.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
استعراض عام
Life consists of reproduction with (heritable) variations.[4] NASA defines life as "a self-sustaining chemical system capable of Darwinian evolution."[5] Such a system is complex; the last universal common ancestor (LUCA), presumably a single-celled organism which lived some 4 billion years ago, already had hundreds of genes encoded in the DNA genetic code that is universal today. That in turn implies a suite of cellular machinery including messenger RNA, transfer RNAs, and ribosomes to translate the code into proteins. Those proteins included enzymes to operate its anaerobic respiration via the Wood–Ljungdahl metabolic pathway, and a DNA polymerase to replicate its genetic material.[6][7]
The challenge for abiogenesis (origin of life)[8][9][10] researchers is to explain how such a complex and tightly-interlinked system could develop by evolutionary steps, as at first sight all its parts are necessary to enable it to function. For example, a cell, whether the LUCA or in a modern organism, copies its DNA with the DNA polymerase enzyme, which is in turn produced by translating the DNA polymerase gene in the DNA. Neither the enzyme nor the DNA can be produced without the other.[11] The evolutionary process could have involved molecular self-replication, self-assembly such as of cell membranes, and autocatalysis.[6][7][12]
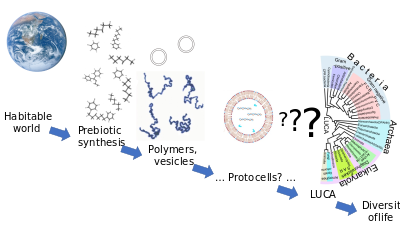
The precursors to the development of a living cell like the LUCA are clear enough, if disputed in their details: a habitable world is formed with a supply of minerals and liquid water. Prebiotic synthesis creates a range of simple organic compounds, which are assembled into polymers such as proteins and RNA. The process after the LUCA, too, is readily understood: Darwinian evolution caused the development of a wide range of species with varied forms and biochemical capabilities. The derivation of living things such as the LUCA from simple components, however, is far from understood.[1]
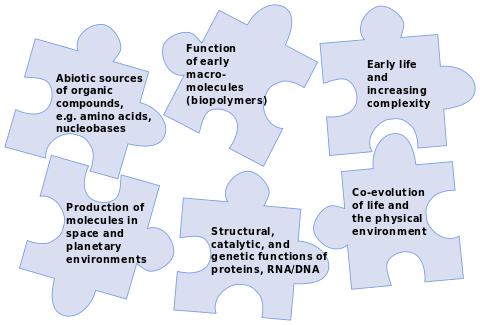
Although Earth remains the only place where life is known,[13][14] the science of astrobiology seeks evidence of life on other planets. The 2015 NASA strategy on the origin of life aimed to solve the puzzle by identifying interactions, intermediary structures and functions, energy sources, and environmental factors that contributed to the diversity, selection, and replication of evolvable macromolecular systems,[3] and mapping the chemical landscape of potential primordial informational polymers. The advent of polymers that could replicate, store genetic information, and exhibit properties subject to selection was, it suggested, most likely a critical step in the emergence of prebiotic chemical evolution.[3] Those polymers derived, in turn, from simple organic compounds such as nucleobases, amino acids and sugars that could have been formed by reactions in the environment.[15][9][16][17] A successful theory of the origin of life must explain how all these chemicals came into being.[18]
تاريخ المفاهيم حتى ع1960
التولد التلقائي
One ancient view of the origin of life, from Aristotle until the 19th century, is of spontaneous generation.[19] This theory held that "lower" animals were generated by decaying organic substances, and that life arose by chance.[20][21] This was questioned from the 17th century, in works like Thomas Browne's Pseudodoxia Epidemica.[22][23] In 1665, Robert Hooke published the first drawings of a microorganism. In 1676, Antonie van Leeuwenhoek drew and described microorganisms, probably protozoa and bacteria.[24] Van Leeuwenhoek disagreed with spontaneous generation, and by the 1680s convinced himself, using experiments ranging from sealed and open meat incubation and the close study of insect reproduction, that the theory was incorrect.[25] In 1668 Francesco Redi showed that no maggots appeared in meat when flies were prevented from laying eggs.[26] By the middle of the 19th century, spontaneous generation was considered disproven.[27][28]
Panspermia
Another ancient idea dating back to Anaxagoras in the 5th century BC is panspermia,[29] the idea that life exists throughout the universe, distributed by meteoroids, asteroids, comets[30] and planetoids.[31] It does not attempt to explain how life originated in itself, but shifts the origin of life on Earth to another heavenly body. The advantage is that life is not required to have formed on each planet it occurs on, but rather in a more limited set of locations (potentially even a single location), and then spread about the galaxy to other star systems via cometary or meteorite impact.[32]
"بِركة صغيرة دافئة": الحساء الأزلي
The idea that life originated from non-living matter in slow stages appeared in Herbert Spencer's 1864–1867 book Principles of Biology, and in William Turner Thiselton-Dyer's 1879 paper "On spontaneous generation and evolution". On 1 February 1871 Charles Darwin wrote about these publications to Joseph Hooker, and set out his own speculation, suggesting that the original spark of life may have begun in a "warm little pond, with all sorts of ammonia and phosphoric salts, light, heat, electricity, &c. [ك], present, that a proteine [ك] compound was chemically formed ready to undergo still more complex changes." Darwin went on to explain that "at the present day such matter would be instantly devoured or absorbed, which would not have been the case before living creatures were formed."[33][34][35]
Alexander Oparin in 1924 and J. B. S. Haldane in 1929 proposed that the first molecules constituting the earliest cells slowly self-organized from a primordial soup.[36] Haldane suggested that the Earth's prebiotic oceans consisted of a "hot dilute soup" in which organic compounds could have formed.[21][37] J. D. Bernal showed that such mechanisms could form most of the necessary molecules for life from inorganic precursors.[38] In 1967, he suggested three "stages": the origin of biological monomers; the origin of biological polymers; and the evolution from molecules to cells.[39][40]
تجربة ميلر-أوري
 مقالة مفصلة: تجربة ميلر-أوري
مقالة مفصلة: تجربة ميلر-أوري
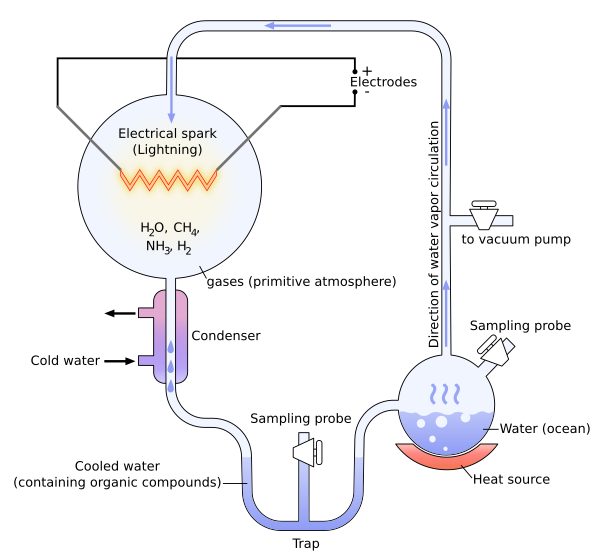
In 1952, Stanley Miller and Harold Urey carried out a chemical experiment to demonstrate how organic molecules could have formed spontaneously from inorganic precursors under prebiotic conditions like those posited by the Oparin-Haldane hypothesis. It used a highly reducing (lacking oxygen) mixture of gases—methane, ammonia, and hydrogen, as well as water vapor—to form simple organic monomers such as amino acids.[41][42] Bernal said of the Miller–Urey experiment that "it is not enough to explain the formation of such molecules, what is necessary, is a physical-chemical explanation of the origins of these molecules that suggests the presence of suitable sources and sinks for free energy."[43] However, current scientific consensus describes the primitive atmosphere as weakly reducing or neutral,[44][45] diminishing the amount and variety of amino acids that could be produced. The addition of iron and carbonate minerals, present in early oceans, however produces a diverse array of amino acids.[44] Later work has focused on two other potential reducing environments: outer space and deep-sea hydrothermal vents.[46][47][48]
إنتاج أرض صالحة للحياة
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
الكون المبكر مع النجوم الأولى
Soon after the Big Bang, which occurred roughly 14 Gya, the only chemical elements present in the universe were hydrogen, helium, and lithium, the three lightest atoms in the periodic table. These elements gradually came together to form stars. These early stars were massive and short-lived, producing all the heavier elements through stellar nucleosynthesis. Carbon, currently the fourth most abundant chemical element in the universe (after hydrogen, helium and oxygen), was formed mainly in white dwarf stars, particularly those bigger than twice the mass of the sun.[49] As these stars reached the end of their lifecycles, they ejected these heavier elements, among them carbon and oxygen, throughout the universe. These heavier elements allowed for the formation of new objects, including rocky planets and other bodies.[50] According to the nebular hypothesis, the formation and evolution of the Solar System began 4.6 Gya with the gravitational collapse of a small part of a giant molecular cloud. Most of the collapsing mass collected in the center, forming the Sun, while the rest flattened into a protoplanetary disk out of which the planets, moons, asteroids, and other small Solar System bodies formed.[51]
ظهور الأرض
The Earth was formed 4.54 Gya.[52][53] The Hadean Earth (from its formation until 4 Gya) was at first inhospitable to any living organisms. During its formation, the Earth lost a significant part of its initial mass, and consequentially lacked the gravity to hold molecular hydrogen and the bulk of the original inert gases.[54] The atmosphere consisted largely of water vapor, nitrogen, and carbon dioxide, with smaller amounts of carbon monoxide, hydrogen, and sulfur compounds.[55] The solution of carbon dioxide in water is thought to have made the seas slightly acidic, with a pH of about 5.5.[56] The Hadean atmosphere has been characterized as a "gigantic, productive outdoor chemical laboratory,"[57] similar to volcanic gases today which still support some abiotic chemistry.[57]
Oceans may have appeared as soon as 200 million years after the Earth formed, in a near-boiling (100 C) reducing environment, as the pH of 5.8 rose rapidly towards neutral.[58] This scenario has found support from the dating of 4.404 Gya zircon crystals from metamorphosed quartzite of Mount Narryer in Western Australia.[59] Despite the likely increased volcanism, the Earth may have been a water world between 4.4 and 4.3 Gya, with little if any continental crust, a turbulent atmosphere, and a hydrosphere subject to intense ultraviolet light from a T Tauri stage Sun, from cosmic radiation, and from continued asteroid and comet impacts.[60]
The Late Heavy Bombardment hypothesis posits that the Hadean environment between 4.28[61] and 3.8 Gya was highly hazardous to life. Following the Nice model, changes in the orbits of the giant planets may have bombarded the Earth with asteroids and comets that pockmarked the Moon and inner planets.[62] Frequent collisions would have made photosynthesis unviable.[57][63][64][65] The periods between such devastating events give time windows for the possible origin of life in early environments. If the deep marine hydrothermal setting was the site for the origin of life, then abiogenesis could have happened as early as 4.0-4.2 Gya. If the site was at the surface of the Earth, abiogenesis could have occurred only between 3.7 and 4.0 Gya.[66] However, new lunar surveys and samples have led scientists, including an architect of the Nice model, to deemphasize the significance of the Late Heavy Bombardment.[67]
If life evolved in the ocean at depths of more than ten meters, it would have been shielded both from late impacts and the then high levels of ultraviolet radiation from the sun. Geothermically heated oceanic crust could have yielded far more organic compounds through deep hydrothermal vents than the Miller–Urey experiments indicated.[68] The available energy is maximized at 100–150 °C, the temperatures at which hyperthermophilic bacteria and thermoacidophilic archaea live. These modern organisms may be among the closest surviving relatives of the LUCA.[69]
أبكر دليل على الحياة
تواجدت الحياة على الأرض لأكثر من 3.5 Gya,[70][71][72] during the Eoarchean when sufficient crust had solidified following the molten Hadean.[73][74][75] The earliest physical evidence of life so far found consists of microfossils in the Nuvvuagittuq Greenstone Belt of Northern Quebec, in banded iron formation rocks at least 3.77 and possibly 4.28 Gya. The micro-organisms lived within hydrothermal vent precipitates, soon after the 4.4 Gya formation of oceans during the Hadean. The microbes resembled modern hydrothermal vent bacteria, supporting the view that abiogenesis began in such an environment.[61]
عـُثِر على گرافيت من أصل حيوي في صخور فوق رسوبية عمرها 3.7 Gya في جنوب غرب گرينلاند[76] and in microbial mat fossils from 3.49 Gya Western Australian sandstone.[77] Evidence of early life in rocks from Akilia Island, near the Isua supracrustal belt in southwestern Greenland, dating to 3.7 Gya, have shown biogenic carbon isotopes.[78] In other parts of the Isua supracrustal belt, graphite inclusions trapped within garnet crystals are connected to the other elements of life: oxygen, nitrogen, and possibly phosphorus in the form of phosphate, providing further evidence for life 3.7 Gya.[79] In the Pilbara region of Western Australia, compelling evidence of early life was found in pyrite-bearing sandstone in a fossilized beach, with rounded tubular cells that oxidized sulfur by photosynthesis in the absence of oxygen.[80][81] Zircons from Western Australia imply that life existed on Earth at least 4.1 Gya.[82]
The Pilbara region of Western Australia contains the Dresser Formation with rocks 3.48 Gya, including layered structures called stromatolites. Their modern counterparts are created by photosynthetic micro-organisms including cyanobacteria.[83] These lie within undeformed hydrothermal-sedimentary strata; their texture indicates a biogenic origin. Parts of the Dresser formation preserve hot springs on land, but other regions seem to have been shallow seas.[84]
Stromatolites in the Siyeh Formation, Glacier National Park, dated 3.5 Gya, placing them among the earliest life-forms.
Modern stromatolites in Shark Bay, created by photosynthetic cyanobacteria.
ظهور الحياة
 مقالة مفصلة: تخلق لاحيوي
مقالة مفصلة: تخلق لاحيوي
حدد جون ماينارد سميث وإيورز سزاثماري ثمانية خطوات رئيسية في الجينات لكي يحدث التطور، ثلاثة منها جعل منا نشك بأن الحياة ظهرت على الأرض [85][86]:
- قيام الحمض النووي RNA World بإنشاء عملية لتخليق البروتين الموجه للتعليمات المشابهة لتخليق البروتين الحديث protein synthesis.
- في وقتاً ما، كل هذه المركبات الكيميائية الحيوية تغلف (تكبسل) بواسطة غشاء (بالرغم من أن التوقيت الدقيق للكبسلة تبقى موضع نزاع بين العلماء)
- تنقل خاصية الحمض النووي لتخزين المعلومات إلى الحمض النووي بطريقة ما بحيث يكون استرجاع المعلومات أسهل.
أقترح جون ديزموند برنال ثلاثة خطوات وهي:
- ظهور المونيمرات العضوية.
- تتحول إلى بوليمرات عضوية.
- تتطور من الجزيئات إلى خلايا.
في الواقع لا يوجد نموذج "موحد" لوصف أصل الحياة، إلا أن النموذج الأكثر شيوعاً وقبولاً والتي تعتمد على الفرضيات العلمية هي :
- بعض الشروط المناسبة التي أتت في ما قبل الحياة، أدت إلى تكون جزيئات عضوية بسيطة والتي هي أساس الحياة.
- تشكلت ليبيدات الفوسفات تلقائياً، والتي هي التركيبة الأساسية للأغشية الخلوية.
- لآليات التي تنتج فيها الحمض النووي RNA (الحمض النووي الريبوزي)، قادرة على إنتاج إنزيمات للحمض النووي RNA في ظروف خاصة جداً لاتتكرر. وكانت هذه أول شكل من أشكال الجينات، والتي تتكون بسببها فيما بعد الخلايا الأولية protocellules.(أنظر فرضية RNA العالم)[87]
- إن أنزيمات RNA تقوم تدريجياً بالاستعاضة عن البروتين والإنزيمات، وذلك بفضل ظهور الريبوزايمات ribozymes (وهي RNA ذو فعالية أنزيمية) وهي قادرة على تخليق البروتين.
- يحل الحمض النووي DNA محل الحمض النووي RNA مدعمةً بالجينات وفي الوقت نفسه تكمل البروتين الرايبوزايمات، مكونةً الرايبوسوم. بظهور هذه التراكيب، بدء التنظيم الحالي للكائنات الحية.
الخطوتين 2 و3 يمكن أن تكونا العكس، حيث أن كلاهما يمكن أن يحدثا بعد التناسخ الذاتي للحمض النووي RNA.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Producing molecules: prebiotic synthesis
All chemical elements except for hydrogen and helium derive from stellar nucleosynthesis. The basic chemical ingredients of life – the carbon-hydrogen molecule (CH), the carbon-hydrogen positive ion (CH+) and the carbon ion (C+) – were produced by ultraviolet light from stars.[88] Complex molecules, including organic molecules, form naturally both in space and on planets.[89] Organic molecules on the early Earth could have had either terrestrial origins, with organic molecule synthesis driven by impact shocks or by other energy sources, such as ultraviolet light, redox coupling, or electrical discharges; or extraterrestrial origins (pseudo-panspermia), with organic molecules formed in interstellar dust clouds raining down on to the planet.[90][91]
Observed extraterrestrial organic molecules
An organic compound is a chemical whose molecules contain carbon. Carbon is abundant in the Sun, stars, comets, and in the atmospheres of most planets.[92] Organic compounds are relatively common in space, formed by "factories of complex molecular synthesis" which occur in molecular clouds and circumstellar envelopes, and chemically evolve after reactions are initiated mostly by ionizing radiation.[89][93][94] Purine and pyrimidine nucleobases including guanine, adenine, cytosine, uracil and thymine have been found in meteorites. These could have provided the materials for DNA and RNA to form on the early Earth.[95] The amino acid glycine was found in material ejected from comet Wild 2; it had earlier been detected in meteorites.[96] Comets are encrusted with dark material, thought to be a tar-like organic substance formed from simple carbon compounds under ionizing radiation. A rain of material from comets could have brought such complex organic molecules to Earth.[97][98][57] It is estimated that during the Late Heavy Bombardment, meteorites may have delivered up to five million tons of organic prebiotic elements to Earth per year.[57]
PAH world hypothesis

Green areas show regions where radiation from hot stars collided with large molecules and small dust grains called "polycyclic aromatic hydrocarbons" (PAHs), causing them to fluoresce.
(Spitzer Space Telescope, 2018)
Polycyclic aromatic hydrocarbons (PAH) are the most common and abundant polyatomic molecules in the observable universe, and are a major store of carbon.[92][99][100][101] They seem to have formed shortly after the Big Bang,[102][100][101] and are associated with new stars and exoplanets.[92] They are a likely constituent of Earth's primordial sea.[102][100][101] PAHs have been detected in nebulae,[103] and in the interstellar medium, in comets, and in meteorites.[92]
The PAH world hypothesis posits PAHs as precursors to the RNA world.[104] A star, HH 46-IR, resembling the sun early in its life, is surrounded by a disk of material which contains molecules including cyanide compounds, hydrocarbons, and carbon monoxide. PAHs in the interstellar medium can be transformed through hydrogenation, oxygenation, and hydroxylation to more complex organic compounds used in living cells.[105]
Nucleobases
The majority of organic compounds introduced on Earth by interstellar dust particles have helped to form complex molecules, thanks to their peculiar surface-catalytic activities.[106][107] Studies of the 12C/13C isotopic ratios of organic compounds in the Murchison meteorite suggest that the RNA component uracil and related molecules, including xanthine, were formed extraterrestrially.[108] NASA studies of meteorites suggest that all four DNA nucleobases (adenine, guanine and related organic molecules) have been formed in outer space.[106][109][110] The cosmic dust permeating the universe contains complex organics ("amorphous organic solids with a mixed aromatic–aliphatic structure") that could be created rapidly by stars.[111] Glycolaldehyde, a sugar molecule and RNA precursor, has been detected in regions of space including around protostars and on meteorites.[112][113]
Laboratory synthesis
As early as the 1860s, experiments demonstrated that biologically relevant molecules can be produced from interaction of simple carbon sources with abundant inorganic catalysts. The spontaneous formation of complex polymers from abiotically generated monomers under the conditions posited by the "soup" theory is not straightforward. Besides the necessary basic organic monomers, compounds that would have prohibited the formation of polymers were also formed in high concentration during the Miller–Urey and Joan Oró experiments.[114] Biology uses essentially 20 amino acids for its coded protein enzymes, representing a very small subset of the structurally possible products. Since life tends to use whatever is available, an explanation is needed for why the set used is so small.[115]
Sugars
Alexander Butlerov showed in 1861 that the formose reaction created sugars including tetroses, pentoses, and hexoses when formaldehyde is heated under basic conditions with divalent metal ions like calcium. R. Breslow proposed that the reaction was autocatalytic in 1959.[116]
Nucleobases
Nucleobases like guanine and adenine can be synthesized from simple carbon and nitrogen sources like hydrogen cyanide (HCN) and ammonia.[117] Formamide produces all four ribonucleotides when warmed with terrestrial minerals. Formamide is ubiquitous in the Universe, produced by the reaction of water and HCN. It can be concentrated by the evaporation of water.[118][119] HCN is poisonous only to aerobic organisms (eukaryotes and aerobic bacteria), which did not yet exist. It can play roles in other chemical processes such as the synthesis of the amino acid glycine.[57]
DNA and RNA components including uracil, cytosine and thymine can be synthesized under outer space conditions, using starting chemicals such as pyrimidine found in meteorites. Pyrimidine may have been formed in red giant stars or in interstellar dust and gas clouds.[120] All four RNA-bases may be synthesized from formamide in high-energy density events like extraterrestrial impacts.[121]
Other pathways for synthesizing bases from inorganic materials have been reported.[122] Freezing temperatures are advantageous for the synthesis of purines, due to the concentrating effect for key precursors such as hydrogen cyanide.[123] However, while adenine and guanine require freezing conditions for synthesis, cytosine and uracil may require boiling temperatures.[124] Seven different amino acids and eleven types of nucleobases formed in ice when ammonia and cyanide were left in a freezer for 25 years.[125][126] S-triazines (alternative nucleobases), pyrimidines including cytosine and uracil, and adenine can be synthesized by subjecting a urea solution to freeze-thaw cycles under a reductive atmosphere, with spark discharges as an energy source.[127] The explanation given for the unusual speed of these reactions at such a low temperature is eutectic freezing, which crowds impurities in microscopic pockets of liquid within the ice, causing the molecules to collide more often.[128]
Producing suitable vesicles
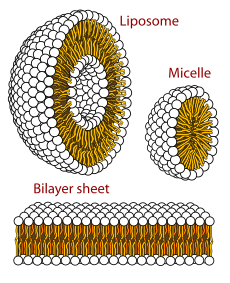
The lipid world theory postulates that the first self-replicating object was lipid-like.[129][130] Phospholipids form lipid bilayers in water while under agitation—the same structure as in cell membranes. These molecules were not present on early Earth, but other amphiphilic long-chain molecules also form membranes. These bodies may expand by insertion of additional lipids, and may spontaneously split into two offspring of similar size and composition. The main idea is that the molecular composition of the lipid bodies is a preliminary to information storage, and that evolution led to the appearance of polymers like RNA that store information. Studies on vesicles from amphiphiles that might have existed in the prebiotic world have so far been limited to systems of one or two types of amphiphiles.[131]
A lipid bilayer membrane could be composed of a huge number of combinations of arrangements of amphiphiles. The best of these would have favored the constitution of a hypercycle,[132][133] actually a positive feedback composed of two mutual catalysts represented by a membrane site and a specific compound trapped in the vesicle. Such site/compound pairs are transmissible to the daughter vesicles leading to the emergence of distinct lineages of vesicles, which would have allowed Darwinian natural selection.[134]
A protocell is a self-organized, self-ordered, spherical collection of lipids proposed as a stepping-stone to the origin of life.[131] The theory of classical irreversible thermodynamics treats self-assembly under a generalized chemical potential within the framework of dissipative systems.[135][136][137]
A central question in evolution is how simple protocells first arose and differed in reproductive contribution to the following generation, thus driving the evolution of life. A functional protocell has (as of 2014) not yet been achieved in a laboratory setting.[138][139][140] Self-assembled vesicles are essential components of primitive cells.[131] The second law of thermodynamics requires that the universe move in a direction in which entropy increases, yet life is distinguished by its great degree of organization. Therefore, a boundary is needed to separate life processes from non-living matter.[141] Irene Chen and Jack W. Szostak suggest that elementary protocells can give rise to cellular behaviors including primitive forms of differential reproduction, competition, and energy storage.[139] Competition for membrane molecules would favor stabilized membranes, suggesting a selective advantage for the evolution of cross-linked fatty acids and even the phospholipids of today.[139] Such micro-encapsulation would allow for metabolism within the membrane and the exchange of small molecules, while retaining large biomolecules inside. Such a membrane is needed for a cell to create its own electrochemical gradient to store energy by pumping ions across the membrane.[142][143]
Producing biology
Energy and entropy
Life requires a loss of entropy, or disorder, when molecules organize themselves into living matter. The emergence of life and increased complexity does not contradict the second law of thermodynamics, which states that overall entropy never decreases, since a living organism creates order in some places (e.g. its living body) at the expense of an increase of entropy elsewhere (e.g. heat and waste production).[144][145][146]
Multiple sources of energy were available for chemical reactions on the early Earth. Heat from geothermal processes is a standard energy source for chemistry. Other examples include sunlight, lightning,[57] atmospheric entries of micro-meteorites,[147] and implosion of bubbles in sea and ocean waves.[148] This has been confirmed by experiments[149][150] and simulations.[151] Unfavorable reactions can be driven by highly favorable ones, as in the case of iron-sulfur chemistry. For example, this was probably important for carbon fixation.[أ] Carbon fixation by reaction of CO2 with H2S via iron-sulfur chemistry is favorable, and occurs at neutral pH and 100 °C. Iron-sulfur surfaces, which are abundant near hydrothermal vents, can drive the production of small amounts of amino acids and other biomolecules.[57]
Chemiosmosis
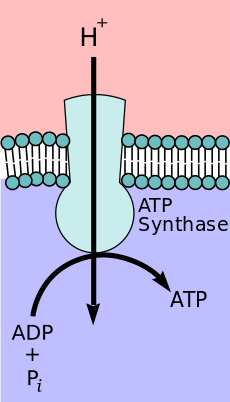
In 1961, Peter Mitchell proposed chemiosmosis as a cell's primary system of energy conversion. The mechanism, now ubiquitous in living cells, powers energy conversion in micro-organisms and in the mitochondria of eukaryotes, making it a likely candidate for early life.[152][153] Mitochondria produce adenosine triphosphate (ATP), the energy currency of the cell used to drive cellular processes such as chemical syntheses. The mechanism of ATP synthesis involves a closed membrane in which the ATP synthase enzyme is embedded. The energy required to release strongly-bound ATP has its origin in protons that move across the membrane.[154] In modern cells, those proton movements are caused by the pumping of ions across the membrane, maintaining an electrochemical gradient. In the first organisms, the gradient could have been provided by the difference in chemical composition between the flow from a hydrothermal vent and the surrounding seawater.[143]
The RNA world
The RNA world hypothesis describes an early Earth with self-replicating and catalytic RNA but no DNA or proteins.[155] Many researchers concur that an RNA world must have preceded the DNA-based life that now dominates.[156] However, RNA-based life may not have been the first to exist.[157][158] Another model echoes Darwin's "warm little pond" with cycles of wetting and drying.[159]
RNA is central to the translation process. Small RNAs can catalyze all the chemical groups and information transfers required for life.[158][160] RNA both expresses and maintains genetic information in modern organisms; and the chemical components of RNA are easily synthesized under the conditions that approximated the early Earth, which were very different from those that prevail today. The structure of the ribozyme has been called the "smoking gun", with a central core of RNA and no amino acid side chains within 18 Å of the active site that catalyzes peptide bond formation.[161][157][162]
The concept of the RNA world was proposed in 1962 by Alexander Rich,[163] and the term was coined by Walter Gilbert in 1986.[158][164] There were initial difficulties in the explanation of the abiotic synthesis of the nucleotides cytosine and uracil.[165] Subsequent research has shown possible routes of synthesis; for example, formamide produces all four ribonucleotides and other biological molecules when warmed in the presence of various terrestrial minerals.[118][119]
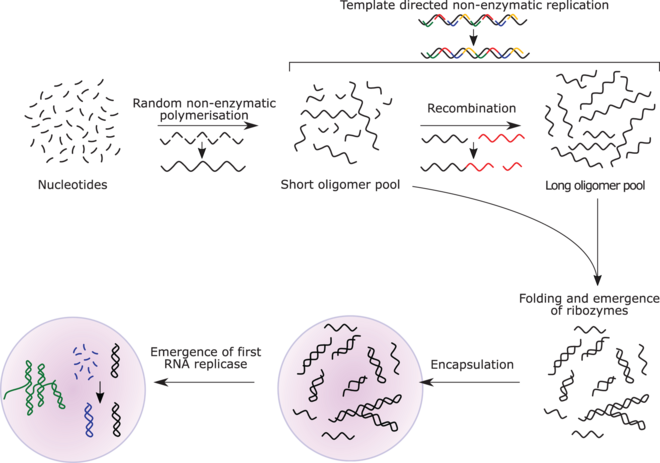
RNA replicase can function as both code and catalyst for further RNA replication, i.e. it can be autocatalytic. Jack Szostak has shown that certain catalytic RNAs can join smaller RNA sequences together, creating the potential for self-replication. The RNA replication systems, which include two ribozymes that catalyze each other's synthesis, showed a doubling time of the product of about one hour, and were subject to Darwinian natural selection under the experimental conditions.[166][167][157] If such conditions were present on early Earth, then natural selection would favor the proliferation of such autocatalytic sets, to which further functionalities could be added.[168][169][170] Self-assembly of RNA may occur spontaneously in hydrothermal vents.[171][172][173] A preliminary form of tRNA could have assembled into such a replicator molecule.[174]
Possible precursors to protein synthesis include the synthesis of short peptide cofactors or the self-catalysing duplication of RNA. It is likely that the ancestral ribosome was composed entirely of RNA, although some roles have since been taken over by proteins. Major remaining questions on this topic include identifying the selective force for the evolution of the ribosome and determining how the genetic code arose.[175]
Eugene Koonin has argued that "no compelling scenarios currently exist for the origin of replication and translation, the key processes that together comprise the core of biological systems and the apparent pre-requisite of biological evolution. The RNA World concept might offer the best chance for the resolution of this conundrum but so far cannot adequately account for the emergence of an efficient RNA replicase or the translation system."[176]
Phylogeny and LUCA
Starting with the work of Carl Woese from 1977 onwards, genomics studies have placed the last universal common ancestor (LUCA) of all modern life-forms between Bacteria and a clade formed by Archaea and Eukaryota in the phylogenetic tree of life. It lived over 4 Gya.[177][178] A minority of studies have placed the LUCA in Bacteria, proposing that Archaea and Eukaryota are evolutionarily derived from within Eubacteria;[179] Thomas Cavalier-Smith suggested that the phenotypically diverse bacterial phylum Chloroflexota contained the LUCA.[180]
Phylogenetic tree showing the last universal common ancestor (LUCA) at the root. The major clades are the Bacteria on one hand, and the Archaea and Eukaryota on the other.
In 2016, a set of 355 genes likely present in the LUCA was identified. A total of 6.1 million prokaryotic genes from Bacteria and Archaea were sequenced, identifying 355 protein clusters from amongst 286,514 protein clusters that were probably common to the LUCA. The results suggest that the LUCA was anaerobic with a Wood–Ljungdahl pathway, nitrogen- and carbon-fixing, thermophilic. Its cofactors suggest dependence upon an environment rich in hydrogen, carbon dioxide, iron, and transition metals. Its genetic material was probably DNA, requiring the 4-nucleotide genetic code, messenger RNA, transfer RNAs, and ribosomes to translate the code into proteins such as enzymes. LUCA likely inhabited an anaerobic hydrothermal vent setting in a geochemically active environment. It was evidently already a complex organism, and must have had precursors; it was not the first living thing.[11][181] The physiology of LUCA has been in dispute.[182][183][184]
LUCA systems and environment[11]
Leslie Orgel argued that early translation machinery for the genetic code would be susceptible to error catastrophe. Geoffrey Hoffmann however showed that such machinery can be stable in function against "Orgel's paradox".[185][186][187]
Suitable geological environments
Deep sea hydrothermal vents

Early micro-fossils may have come from a hot world of gases such as methane, ammonia, carbon dioxide and hydrogen sulphide, toxic to much current life.[188] Analysis of the tree of life places thermophilic and hyperthermophilic bacteria and archaea closest to the root, suggesting that life may have evolved in a hot environment.[189] The deep sea or alkaline hydrothermal vent theory posits that life began at submarine hydrothermal vents.[190][191] Martin and Russell have suggested "that life evolved in structured iron monosulphide precipitates in a seepage site hydrothermal mound at a redox, pH, and temperature gradient between sulphide-rich hydrothermal fluid and iron(II)-containing waters of the Hadean ocean floor. The naturally arising, three-dimensional compartmentation observed within fossilized seepage-site metal sulphide precipitates indicates that these inorganic compartments were the precursors of cell walls and membranes found in free-living prokaryotes. The known capability of FeS and NiS to catalyze the synthesis of the acetyl-methylsulphide from carbon monoxide and methylsulphide, constituents of hydrothermal fluid, indicates that pre-biotic syntheses occurred at the inner surfaces of these metal-sulphide-walled compartments".[192]
These form where hydrogen-rich fluids emerge from below the sea floor, as a result of serpentinization of ultra-mafic olivine with seawater and a pH interface with carbon dioxide-rich ocean water. The vents form a sustained chemical energy source derived from redox reactions, in which electron donors (molecular hydrogen) react with electron acceptors (carbon dioxide); see Iron–sulfur world theory. These are exothermic reactions.[190][ب]
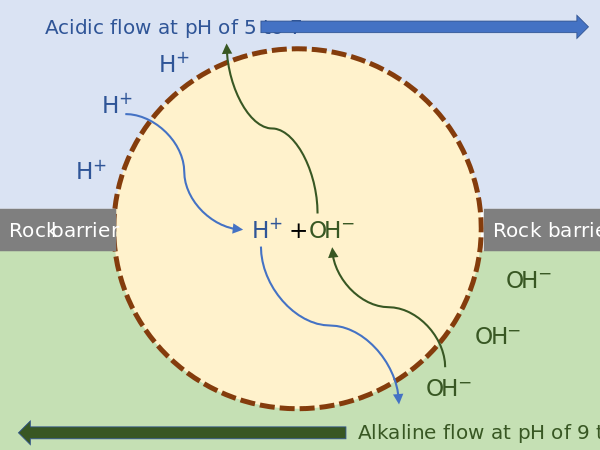
Russell demonstrated that alkaline vents created an abiogenic proton motive force chemiosmotic gradient,[192] ideal for abiogenesis. Their microscopic compartments "provide a natural means of concentrating organic molecules," composed of iron-sulfur minerals such as mackinawite, endowed these mineral cells with the catalytic properties envisaged by Günter Wächtershäuser.[193] This movement of ions across the membrane depends on a combination of two factors:
- Diffusion force caused by concentration gradient—all particles including ions tend to diffuse from higher concentration to lower.
- Electrostatic force caused by electrical potential gradient—cations like protons H+ tend to diffuse down the electrical potential, anions in the opposite direction.
These two gradients taken together can be expressed as an electrochemical gradient, providing energy for abiogenic synthesis. The proton motive force can be described as the measure of the potential energy stored as a combination of proton and voltage gradients across a membrane (differences in proton concentration and electrical potential).[143]
The surfaces of mineral particles inside deep-ocean hydrothermal vents have catalytic properties similar to those of enzymes and can create simple organic molecules, such as methanol (CH3OH), formic, acetic and pyruvic acids out of the dissolved CO2 in the water, if driven by an applied voltage or by reaction with H2 or H2S.[194][195]
The research reported by Martin in 2016 supports the thesis that life arose at hydrothermal vents,[196][197] that spontaneous chemistry in the Earth's crust driven by rock–water interactions at disequilibrium thermodynamically underpinned life's origin[198][199] and that the founding lineages of the archaea and bacteria were H2-dependent autotrophs that used CO2 as their terminal acceptor in energy metabolism.[200] Martin suggests, based upon this evidence, that the LUCA "may have depended heavily on the geothermal energy of the vent to survive".[201] Colín-García and colleagues argue that exergonic reactions in hydrothermal vents could provide free energy to promote chemical reactions, and many different minerals. These induce chemical gradients, favoring the interaction between electron donors and acceptors.[202]
Hot springs
Mulkidjanian and co-authors think that the marine environments did not provide the ionic balance and composition universally found in cells, or the ions required by essential proteins and ribozymes found in virtually all living organisms, especially with respect to K+/Na+ ratio, Mn2+, Zn2+ and phosphate concentrations. They argue that the only environments that mimic the needed conditions on Earth are hot springs.[203] Mineral deposits in these environments under an anoxic atmosphere would have suitable pH (while current pools in an oxygenated atmosphere would not), contain precipitates of sulfide minerals that absorb harmful ultraviolet radiation, have wetting/drying cycles that concentrate substrate solutions to concentrations amenable to spontaneous formation of polymers of nucleic acids, polyesters[204] and depsipeptides,[205] created both by chemical reactions in the hydrothermal environment, and by exposure to UV light during transport from vents to adjacent pools.[206] The hypothesized pre-biotic environments are similar to deep-oceanic vent environments, with additional components that help explain peculiarities of the LUCA.[207][208] Experimental research at hot springs supports this hypothesis, as RNA-like polymers were synthesized after multiple wet-dry cycles and exposure to UV light. These polymers became encapsulated in vesicles after rehydration, which would not happen in saltwater conditions.[209]
Clay
The clay hypothesis was proposed by Graham Cairns-Smith in 1985.[210][211] It postulates that complex organic molecules arose gradually on pre-existing, non-organic replication surfaces of silicate crystals in contact with an aqueous solution. The clay mineral montmorillonite has been shown to catalyze the polymerization of RNA in aqueous solution from nucleotide monomers,[212] and the formation of membranes from lipids.[213] In 1998, Hyman Hartman proposed that "the first organisms were self-replicating iron-rich clays which fixed carbon dioxide into oxalic acid and other dicarboxylic acids. This system of replicating clays and their metabolic phenotype then evolved into the sulfide rich region of the hotspring acquiring the ability to fix nitrogen. Finally phosphate was incorporated into the evolving system which allowed the synthesis of nucleotides and phospholipids."[214]
Iron–sulfur world
In the 1980s, Günter Wächtershäuser and Karl Popper postulated the Iron–sulfur world hypothesis for the evolution of pre-biotic chemical pathways. It traces today's biochemistry to primordial reactions which synthesize organic building blocks from gases.[215][216] Wächtershäuser systems have a built-in source of energy: iron sulfides such as pyrite. The energy released by oxidising these metal sulfides can support synthesis of organic molecules. Such systems may have evolved into autocatalytic sets constituting self-replicating, metabolically active entities predating modern life forms.[217] Experiments with sulfides in an aqueous environment at 100 °C produced a small yield of dipeptides (0.4% to 12.4%) and a smaller yield of tripeptides (0.10%). However, under the same conditions, dipeptides were quickly broken down.[218]
Several models postulate a primitive metabolism, allowing RNA replication to emerge later. The centrality of the Krebs cycle (citric acid cycle) to energy production in aerobic organisms, and in drawing in carbon dioxide and hydrogen ions in biosynthesis of complex organic chemicals, suggests that it was one of the first parts of the metabolism to evolve.[193] Concordantly, geochemists Jack W. Szostak and Kate Adamala demonstrated that non-enzymatic RNA replication in primitive protocells is only possibly in the presence of weak cation chelators like citric acid. This provides further evidence for the central role of citric acid in primordial metabolism.[219] Russell has proposed that "the purpose of life is to hydrogenate carbon dioxide" (as part of a "metabolism-first," rather than a "genetics-first," scenario).[220][221][217] The physicist Jeremy England has argued from general thermodynamic considerations that life was inevitable.[222] An early version of this idea was Oparin's 1924 proposal for self-replicating vesicles. In the 1980s and 1990s came Wächtershäuser's iron–sulfur world theory and Christian de Duve's thioester models. More abstract and theoretical arguments for metabolism without genes include Freeman Dyson's mathematical model and Stuart Kauffman's collectively autocatalytic sets in the 1980s. Kauffman's work has been criticized for ignoring the role of energy in driving biochemical reactions in cells.[223]
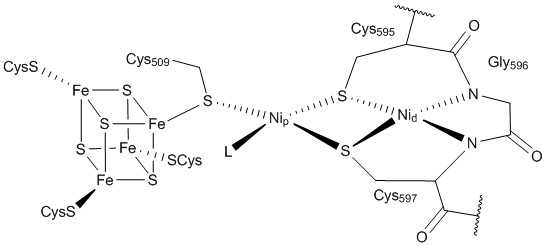
A multistep biochemical pathway like the Krebs cycle did not simply self-organize on the surface of a mineral; it must have been preceded by simpler pathways.[224] The Wood–Ljungdahl pathway is compatible with self-organization on a metal sulfide surface. Its key enzyme unit, carbon monoxide dehydrogenase/acetyl-CoA synthase, contains mixed nickel-iron-sulfur clusters in its reaction centers and catalyzes the formation of acetyl-CoA. However, prebiotic thiolated and thioester compounds are thermodynamically and kinetically unlikely to accumulate in the presumed prebiotic conditions of hydrothermal vents.[225] One possibility is that cysteine and homocysteine may have reacted with nitriles from the Stecker reaction, forming catalytic thiol-rich polypeptides.[226]
Zinc-world
Armen Mulkidjanian's zinc world (Zn-world) hypothesis extends Wächtershäuser's pyrite hypothesis.[227][228] The Zn-world theory proposes that hydrothermal fluids rich in H2S interacting with cold primordial ocean (or Darwin's "warm little pond") water precipitated metal sulfide particles. Oceanic hydrothermal systems have a zonal structure reflected in ancient volcanogenic massive sulfide ore deposits. They reach many kilometers in diameter and date back to the Archean. Most abundant are pyrite (FeS2), chalcopyrite (CuFeS2), and sphalerite (ZnS), with additions of galena (PbS) and alabandite (MnS). ZnS and MnS have a unique ability to store radiation energy, e.g. from ultraviolet light. When replicating molecules were originating, the primordial atmospheric pressure was high enough (>100 bar) to precipitate near the Earth's surface, and ultraviolet irradiation was 10 to 100 times more intense than now; hence the photosynthetic properties mediated by ZnS provided the right energy conditions for the synthesis of informational and metabolic molecules and the selection of photostable nucleobases.[227][229]
The Zn-world theory has been filled out with evidence for the ionic constitution of the interior of the first proto-cells. In 1926, the Canadian biochemist Archibald Macallum noted the resemblance of body fluids such as blood and lymph to seawater;[230] however, the inorganic composition of all cells differ from that of modern seawater, which led Mulkidjanian and colleagues to reconstruct the "hatcheries" of the first cells combining geochemical analysis with phylogenomic scrutiny of the inorganic ion requirements of modern cells. The authors conclude that ubiquitous, and by inference primordial, proteins and functional systems show affinity to and functional requirement for K+, Zn2+, Mn2+, and [PO 4]3− . Geochemical reconstruction shows that this ionic composition could not have existed in the ocean but is compatible with inland geothermal systems. In the oxygen-depleted, CO2-dominated primordial atmosphere, the chemistry of water condensates near geothermal fields would resemble the internal milieu of modern cells. Therefore, precellular evolution may have taken place in shallow "Darwin ponds" lined with porous silicate minerals mixed with metal sulfides and enriched in K+, Zn2+, and phosphorus compounds.[231][232]
Homochirality

Homochirality is the geometric uniformity of materials composed of chiral (non-mirror-symmetric) units. Living organisms use molecules that have the same chirality (handedness): with almost no exceptions,[234] amino acids are left-handed while nucleotides and sugars are right-handed. Chiral molecules can be synthesized, but in the absence of a chiral source or a chiral catalyst, they are formed in a 50/50 (racemic) mixture of both forms. Known mechanisms for the production of non-racemic mixtures from racemic starting materials include: asymmetric physical laws, such as the electroweak interaction; asymmetric environments, such as those caused by circularly polarized light, quartz crystals, or the Earth's rotation, statistical fluctuations during racemic synthesis,[233] and spontaneous symmetry breaking.[235][236][237] Once established, chirality would be selected for.[238] A small bias (enantiomeric excess) in the population can be amplified into a large one by asymmetric autocatalysis, such as in the Soai reaction.[239] In asymmetric autocatalysis, the catalyst is a chiral molecule, which means that a chiral molecule is catalyzing its own production. An initial enantiomeric excess, such as can be produced by polarized light, then allows the more abundant enantiomer to outcompete the other.[240] Homochirality may have started in outer space, as on the Murchison meteorite the amino acid L-alanine is more than twice as frequent as its D form, and L-glutamic acid is more than three times as abundant as its D counterpart.[241][242] Amino acids from meteorites show a left-handed bias, whereas sugars show a predominantly right-handed bias, as found in living organisms, suggesting an abiogenic origin of these compounds.[243]
النطفة الفضائية
النطفة الفضائية هي فرضية تقول بأن حبوب الحياة كانت موجودة مسبقاً منذ نشوء الكون، وأنها وصلت إلى كوكب الأرض وو بدأت بالارتقاء بسبب قابليتها للحياة. فرانسيس كريك هو من أشهر مؤيدي هذه الفرضية [244] وقد وضع نظرية البانسبيرميا الموجهة Directed panspermia التي تقول بإنه يمكن حساب احتمالية إصابة البذور لهدف معين من حيث أن A(target) هو مساحة المقطع العرضي للهدف، وdy هي قيمة عدم اليقين للمكان الذي سيصل إليه البذور، وa هو ثابت (يعتمد على الوحدات الموجودة)، وr(target) هو نصف قطر لمساحة الهدف، وvهو سرعة المسبار -أو المذنب- الذي ينقل البذور، و(tp) هي قيمة دقة الاستهداف، وd هي بعدها عن الهدف.[245]
نظريات حديثة
في مارس 2015، طرح علماء الكيمياء من مجلس الأبحاث الطبية وجامعة كمبردج نظرية جديدة بشأن نشوء الحياة على الأرض، استنادا الى كافة الاكتشافات والدراسات السابقة، مع الأخذ بالاعتبار رأي كافة الأطراف التي ابدت رأيها في هذا المجال.
وصف علماء الكيمياء العمليات التي سبقت تشكيل وحدات "لبنات" البناء عند نشوء الحياة على الأرض، واعتبروها عمليات كيميائية بفضلها ظهرت السكريات والأحماض الأمينية وريبونوكليتويدات والگليسرين ثنائية وثلاثية الكربون. أي المكونات الأساسية لعمليات الأيض (التمثيل الغذائي). وقد تمكنوا من اعادة عملية نشوء الدهون التي تشكل اغشية الخلايا في الأحياء المعاصرة.[246]
واعتماداً على العينات ونتائج التجارب التي أجروها، يقول علماء الكيمياء، أن مكونات الحياة يمكن ان تظهر من كبريتيد الهيدروجين وحمض سيان الماء "حمض البروسيك" تحت تأثير الأشعة فوق البنفسجية. لأن هذا الحمض يتفاعل بسهولة مع كبريتيد الهيدروجين، ويتسارع هذا التفاعل بوجود اشعة الشمس، التي كانت متوفرة عند نشوء الأشكال البدائية للحياة على الأرض.
ويذكر ان العلماء ينقسمون الى ثلاثة مجموعات. المجموعة الأولى تقول انه لنشوء الحياة على الأرض كان يجب أن يسبقه تكون جزيئات الحمض النووي الريبوزي "RNA ". المجموعة الثانية من العلماء فتقول، لا بد ان يسبق نشوء الحياة على الأرض تطور عمليات الأيض. أما المجموعة الثالثة فتقول، كان لا بد من ظهور اغشية الخلايا البدائية.
يشير علماء الكيمياء الى أنهم استندوا الى هذه الفرضيات الثلاث في دراستهم، وهم في انتظار ردود فعل العلماء الآخرين على نتائجها. فقد تصبح الأساس في نظرية جديدة عن نشوء الحياة على الأرض.
انظر أيضاً
المراجع
Explanatory footnotes
- ^ The reactions are:
- FeS + H2S → FeS2 + 2H+ + 2e−
- FeS + H2S + CO2 → FeS2 + HCOOH
- ^ The reactions are:
Reaction 1: Fayalite + water → magnetite + aqueous silica + hydrogen- 3Fe2SiO4 + 2H2O → 2Fe3O4 + 3SiO2 + 2H2
- 3Mg2SiO4 + SiO2 + 4H2O → 2Mg3Si2O5(OH)4
- 2Mg2SiO4 + 3H2O → Mg3Si2O5(OH)4 + Mg(OH)2
- 2 Ca2SiO4 + 4 H2O → 3 CaO · 2 SiO2 · 3 H2O + Ca(OH)2
Citations
- ^ أ ب Walker, Sara I.; Packard, N.; Cody, G. D. (13 November 2017). "Re-conceptualizing the origins of life". Philosophical Transactions of the Royal Society A. 375 (2109): 20160337. Bibcode:2017RSPTA.37560337W. doi:10.1098/rsta.2016.0337. PMC 5686397. PMID 29133439.
- ^ J.B. Bernal, in M. Florkin (22 October 2013). "Problem of the stages in biopoesis". Aspects of the Origin of Life: International Series of Monographs on Pure and Applied Biology. Elsevier. pp. 30–. ISBN 978-1-4831-3587-8.
- ^ أ ب ت "NASA Astrobiology Strategy" (PDF). NASA. 2015. Archived from the original (PDF) on 22 December 2016. Retrieved 24 September 2017.
- ^ Trifonov, Edward N. (17 March 2011). "Vocabulary of Definitions of Life Suggests a Definition". Journal of Biomolecular Structure and Dynamics. 29 (2): 259–266. doi:10.1080/073911011010524992. PMID 21875147. S2CID 38476092.
- ^ Voytek, Mary A. (6 March 2021). "About Life Detection". NASA. Retrieved 8 March 2021.
- ^ أ ب Witzany, Guenther (2016). "Crucial steps to life: From chemical reactions to code using agents" (PDF). BioSystems. 140: 49–57. doi:10.1016/j.biosystems.2015.12.007. PMID 26723230. S2CID 30962295.
- ^ أ ب Howell, Elizabeth (8 December 2014). "How Did Life Become Complex, And Could It Happen Beyond Earth?". Astrobiology Magazine. Archived from the original on 15 February 2018. Retrieved 14 April 2022.
- ^ Oparin, Aleksandr Ivanovich (2003) [1938]. The Origin of Life. Translated by Morgulis, Sergius (2 ed.). Mineola, New York: Courier. ISBN 978-0486495224.
- ^ أ ب Peretó, Juli (2005). "Controversies on the origin of life" (PDF). International Microbiology. 8 (1): 23–31. PMID 15906258. Archived from the original (PDF) on 24 August 2015. Retrieved 1 June 2015.
- ^ Compare: Scharf, Caleb; et al. (18 December 2015). "A Strategy for Origins of Life Research". Astrobiology. 15 (12): 1031–1042. Bibcode:2015AsBio..15.1031S. doi:10.1089/ast.2015.1113. PMC 4683543. PMID 26684503.
What do we mean by the origins of life (OoL)? ... Since the early 20th century the phrase OoL has been used to refer to the events that occurred during the transition from non-living to living systems on Earth, i.e., the origin of terrestrial biology (Oparin, 1924; Haldane, 1929). The term has largely replaced earlier concepts such as abiogenesis (Kamminga, 1980; Fry, 2000).
- ^ أ ب ت Weiss, M. C.; Sousa, F. L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. (2016). "The physiology and habitat of the last universal common ancestor" (PDF). Nature Microbiology. 1 (9): 16116. doi:10.1038/NMICROBIOL.2016.116. PMID 27562259. S2CID 2997255.
- ^ Tirard, Stephane (20 April 2015). Abiogenesis – Definition. p. 1. doi:10.1007/978-3-642-27833-4_2-4. ISBN 978-3-642-27833-4.
Thomas Huxley (1825–1895) used the term abiogenesis in an important text published in 1870. He strictly made the difference between spontaneous generation, which he did not accept, and the possibility of the evolution of matter from inert to living, without any influence of life. ... Since the end of the nineteenth century, evolutive abiogenesis means increasing complexity and evolution of matter from inert to living state in the abiotic context of evolution of primitive Earth.
{{cite book}}:|journal=ignored (help) - ^ Graham, Robert W. (February 1990). "Extraterrestrial Life in the Universe" (PDF). NASA (NASA Technical Memorandum 102363). Lewis Research Center, Cleveland, Ohio. Archived (PDF) from the original on 3 September 2014. Retrieved 2015-06-02.
- ^ Altermann 2009, p. xvii
- ^ Oparin 1953, p. vi
- ^ Warmflash, David; Warmflash, Benjamin (November 2005). "Did Life Come from Another World?". Scientific American. 293 (5): 64–71. Bibcode:2005SciAm.293e..64W. doi:10.1038/scientificamerican1105-64. PMID 16318028.
- ^ Yarus 2010, p. 47
- ^ Ward, Peter; Kirschvink, Joe (2015). A New History of Life: the radical discoveries about the origins and evolution of life on earth. Bloomsbury Press. pp. 39–40. ISBN 978-1608199105.
- ^ Sheldon 2005
- ^ Lennox 2001, pp. 229–258
- ^ أ ب Bernal 1967
- ^ Balme, D. M. (1962). "Development of Biology in Aristotle and Theophrastus: Theory of Spontaneous Generation". Phronesis. 7 (1–2): 91–104. doi:10.1163/156852862X00052.
- ^ Ross 1652
- ^ Dobell 1960
- ^ Bondeson 1999
- ^ Levine, R.; Evers, C. "The Slow Death of Spontaneous Generation (1668-1859)". Archived from the original on 26 April 2008. Retrieved 18 April 2013.
- ^ Oparin 1953, p. 196
- ^ Tyndall 1905, IV, XII (1876), XIII (1878)
- ^ Horneck, Gerda; Klaus, David M.; Mancinelli, Rocco L. (March 2010). "Space Microbiology". Microbiology and Molecular Biology Reviews. 74 (1): 121–156. Bibcode:2010MMBR...74..121H. doi:10.1128/MMBR.00016-09. PMC 2832349. PMID 20197502.
- ^ Wickramasinghe, Chandra (2011). "Bacterial morphologies supporting cometary panspermia: a reappraisal". International Journal of Astrobiology. 10 (1): 25–30. Bibcode:2011IJAsB..10...25W. CiteSeerX 10.1.1.368.4449. doi:10.1017/S1473550410000157. S2CID 7262449.
- ^ Rampelotto, P. H. (2010). "Panspermia: A promising field of research". In: Astrobiology Science Conference. Abs 5224.
- ^ Chang, Kenneth (12 September 2016). "Visions of Life on Mars in Earth's Depths". The New York Times. Archived from the original on 12 September 2016. Retrieved 12 September 2016.
- ^ "Letter no. 7471, Charles Darwin to Joseph Dalton Hooker, 1 February (1871)". Darwin Correspondence Project. Retrieved 7 July 2020.
- ^ Priscu, John C. "Origin and Evolution of Life on a Frozen Earth". Arlington County, Virginia: National Science Foundation. Archived from the original on 18 December 2013. Retrieved 1 March 2014.
- ^ Marshall, Michael (11 November 2020). "Charles Darwin's hunch about early life was probably right". BBC News. Retrieved 11 November 2020.
- ^ Bahadur, Krishna (1973). "Photochemical Formation of Self–sustaining Coacervates" (PDF). Proceedings of the Indian National Science Academy. 39 (4): 455–467. doi:10.1016/S0044-4057(75)80076-1. PMID 1242552. Archived from the original (PDF) on 19 October 2013.
- Bahadur, Krishna (1975). "Photochemical Formation of Self-Sustaining Coacervates". Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. 130 (3): 211–218. doi:10.1016/S0044-4057(75)80076-1. OCLC 641018092. PMID 1242552.
- ^ Bryson 2004, pp. 300–302
- ^ Bernal 1951
- ^ Martin, William F. (January 2003). "On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells". Phil. Trans. R. Soc. Lond. A. 358 (1429): 59–83. doi:10.1098/rstb.2002.1183. PMC 1693102. PMID 12594918.
- ^ Bernal, John Desmond (September 1949). "The Physical Basis of Life". Proceedings of the Physical Society, Section A. 62 (9): 537–558. Bibcode:1949PPSA...62..537B. doi:10.1088/0370-1298/62/9/301. S2CID 83754271.
- ^ Miller, Stanley L. (15 May 1953). "A Production of Amino Acids Under Possible Primitive Earth Conditions". Science. 117 (3046): 528–529. Bibcode:1953Sci...117..528M. doi:10.1126/science.117.3046.528. PMID 13056598.
- ^ Parker, Eric T.; Cleaves, Henderson J.; Dworkin, Jason P.; et al. (5 April 2011). "Primordial synthesis of amines and amino acids in a 1958 Miller H2S-rich spark discharge experiment". PNAS. 108 (14): 5526–5531. Bibcode:2011PNAS..108.5526P. doi:10.1073/pnas.1019191108. PMC 3078417. PMID 21422282.
- ^ Bernal 1967, p. 143
- ^ أ ب Cleaves, H. James; Chalmers, John H.; Lazcano, Antonio; et al. (April 2008). "A Reassessment of Prebiotic Organic Synthesis in Neutral Planetary Atmospheres". Origins of Life and Evolution of Biospheres. 38 (2): 105–115. Bibcode:2008OLEB...38..105C. doi:10.1007/s11084-007-9120-3. PMID 18204914. S2CID 7731172.
- ^ Chyba, Christopher F. (13 May 2005). "Rethinking Earth's Early Atmosphere". Science. 308 (5724): 962–963. doi:10.1126/science.1113157. PMID 15890865. S2CID 93303848.
- ^ Barton et al. 2007, pp. 93–95
- ^ Bada & Lazcano 2009, pp. 56–57
- ^ Bada, Jeffrey L.; Lazcano, Antonio (2 May 2003). "Prebiotic Soup – Revisiting the Miller Experiment" (PDF). Science. 300 (5620): 745–746. doi:10.1126/science.1085145. PMID 12730584. S2CID 93020326. Archived (PDF) from the original on 4 March 2016. Retrieved 2015-06-13.
- ^ Marigo, Paola; et al. (6 July 2020). "Carbon star formation as seen through the non-monotonic initial–final mass relation". Nature Astronomy. 152 (11): 1102–1110. arXiv:2007.04163. Bibcode:2020NatAs...4.1102M. doi:10.1038/s41550-020-1132-1. S2CID 220403402.
- ^ "WMAP- Life in the Universe".
- ^ "Formation of Solar Systems: Solar Nebular Theory". University of Massachusetts Amherst. Retrieved 27 September 2019.
- ^ "Age of the Earth". United States Geological Survey. 9 July 2007. Archived from the original on 23 December 2005. Retrieved 10 January 2006.
- ^ Dalrymple 2001, pp. 205–221
- ^ Fesenkov 1959, p. 9
- ^ Kasting, James F. (12 February 1993). "Earth's Early Atmosphere" (PDF). Science. 259 (5097): 920–926. Bibcode:1993Sci...259..920K. doi:10.1126/science.11536547. PMID 11536547. S2CID 21134564. Archived from the original (PDF) on 10 October 2015. Retrieved 2015-07-28.
- ^ Morse, John (September 1998). "Hadean Ocean Carbonate Geochemistry". Aquatic Geochemistry. 4 (3/4): 301–319. Bibcode:1998MinM...62.1027M. doi:10.1023/A:1009632230875. S2CID 129616933.
- ^ أ ب ت ث ج ح خ د Follmann, Hartmut; Brownson, Carol (November 2009). "Darwin's warm little pond revisited: from molecules to the origin of life". Naturwissenschaften. 96 (11): 1265–1292. Bibcode:2009NW.....96.1265F. doi:10.1007/s00114-009-0602-1. PMID 19760276. S2CID 23259886.
- ^ Morse, John W.; MacKenzie, Fred T. (1998). "Hadean Ocean Carbonate Geochemistry". Aquatic Geochemistry. 4 (3–4): 301–319. Bibcode:1998MinM...62.1027M. doi:10.1023/A:1009632230875. S2CID 129616933.
- ^ Wilde, Simon A.; Valley, John W.; Peck, William H.; Graham, Colin M. (11 January 2001). "Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago" (PDF). Nature. 409 (6817): 175–178. Bibcode:2001Natur.409..175W. doi:10.1038/35051550. PMID 11196637. S2CID 4319774. Archived (PDF) from the original on 5 June 2015. Retrieved 3 June 2015.
- ^ Rosing, Minik T.; Bird, Dennis K.; Sleep, Norman H.; et al. (22 March 2006). "The rise of continents – An essay on the geologic consequences of photosynthesis". Palaeogeography, Palaeoclimatology, Palaeoecology. 232 (2–4): 99–113. Bibcode:2006PPP...232...99R. doi:10.1016/j.palaeo.2006.01.007. Archived (PDF) from the original on 14 July 2015. Retrieved 2015-06-08.
- ^ أ ب ت Dodd, Matthew S.; Papineau, Dominic; Grenne, Tor; et al. (1 March 2017). "Evidence for early life in Earth's oldest hydrothermal vent precipitates". Nature. 543 (7643): 60–64. Bibcode:2017Natur.543...60D. doi:10.1038/nature21377. PMID 28252057. Archived from the original on 8 September 2017. Retrieved 2 March 2017.
- ^ Gomes, Rodney; Levison, Hal F.; Tsiganis, Kleomenis; Morbidelli, Alessandro (26 May 2005). "Origin of the cataclysmic Late Heavy Bombardment period of the terrestrial planets". Nature. 435 (7041): 466–469. Bibcode:2005Natur.435..466G. doi:10.1038/nature03676. PMID 15917802.
- ^ Sleep, Norman H.; Zahnle, Kevin J.; Kasting, James F.; et al. (9 November 1989). "Annihilation of ecosystems by large asteroid impacts on early Earth". Nature. 342 (6246): 139–142. Bibcode:1989Natur.342..139S. doi:10.1038/342139a0. PMID 11536616. S2CID 1137852.
- ^ Chyba, Christopher; Sagan, Carl (9 January 1992). "Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life". Nature. 355 (6356): 125–132. Bibcode:1992Natur.355..125C. doi:10.1038/355125a0. PMID 11538392. S2CID 4346044.
- ^ Furukawa, Yoshihiro; Sekine, Toshimori; Oba, Masahiro; et al. (January 2009). "Biomolecule formation by oceanic impacts on early Earth". Nature Geoscience. 2 (1): 62–66. Bibcode:2009NatGe...2...62F. doi:10.1038/NGEO383.
- ^ Maher, Kevin A.; Stevenson, David J. (18 February 1988). "Impact frustration of the origin of life". Nature. 331 (6157): 612–614. Bibcode:1988Natur.331..612M. doi:10.1038/331612a0. PMID 11536595. S2CID 4284492.
- ^ Mann, Adam (24 January 2018). "Bashing holes in the tale of Earth's troubled youth". Nature. 553 (7689): 393–395. Bibcode:2018Natur.553..393M. doi:10.1038/d41586-018-01074-6.
- ^ Davies 1999, p. 155
- ^ Bock & Goode 1996
- ^ Schopf, J. William; Kudryavtsev, Anatoliy B.; Czaja, Andrew D.; Tripathi, Abhishek B. (5 October 2007). "Evidence of Archean life: Stromatolites and microfossils". Precambrian Research. 158 (3–4): 141–155. Bibcode:2007PreR..158..141S. doi:10.1016/j.precamres.2007.04.009.
- ^ Schopf, J. William (29 June 2006). "Fossil evidence of Archaean life". Philosophical Transactions of the Royal Society B. 361 (1470): 869–885. doi:10.1098/rstb.2006.1834. PMC 1578735. PMID 16754604.
- ^ Raven & Johnson 2002, p. 68
- ^ Djokic, Tara; Van Kranendonk, Martin J.; Campbell, Kathleen A.; Walter, Malcolm R.; Ward, Colin R. (9 May 2017). "Earliest signs of life on land preserved in ca. 3.5 Gao hot spring deposits". Nature Communications. 8: 15263. Bibcode:2017NatCo...815263D. doi:10.1038/ncomms15263. PMC 5436104. PMID 28486437.
- ^ Schopf, J. William; Kitajima, Kouki; Spicuzza, Michael J.; Kudryavtsev, Anatolly B.; Valley, John W. (2017). "SIMS analyses of the oldest known assemblage of microfossils document their taxon-correlated carbon isotope compositions". PNAS. 115 (1): 53–58. Bibcode:2018PNAS..115...53S. doi:10.1073/pnas.1718063115. PMC 5776830. PMID 29255053.
- ^ Tyrell, Kelly April (18 December 2017). "Oldest fossils ever found show life on Earth began before 3.5 billion years ago". University of Wisconsin-Madison. Retrieved 18 December 2017.
- ^ Ohtomo, Yoko; Kakegawa, Takeshi; Ishida, Akizumi; et al. (January 2014). "Evidence for biogenic graphite in early Archaean Isua metasedimentary rocks". Nature Geoscience. 7 (1): 25–28. Bibcode:2014NatGe...7...25O. doi:10.1038/ngeo2025.
- ^ Noffke, Nora; Christian, Daniel; Wacey, David; Hazen, Robert M. (16 November 2013). "Microbially Induced Sedimentary Structures Recording an Ancient Ecosystem in the ca. 3.48 Gyo Dresser Formation, Pilbara, Western Australia". Astrobiology. 13 (12): 1103–1124. Bibcode:2013AsBio..13.1103N. doi:10.1089/ast.2013.1030. PMC 3870916. PMID 24205812.
- ^ Davies 1999
- ^ Hassenkam, T.; Andersson, M. P.; Dalby, K. N.; Mackenzie, D.M.A.; Rosing, M.T. (2017). "Elements of Eoarchean life trapped in mineral inclusions". Nature. 548 (7665): 78–81. Bibcode:2017Natur.548...78H. doi:10.1038/nature23261. PMID 28738409. S2CID 205257931.
- ^ O'Donoghue, James (21 August 2011). "Oldest reliable fossils show early life was a beach". New Scientist. 211: 13. doi:10.1016/S0262-4079(11)62064-2. Archived from the original on 30 June 2015.
- ^ Wacey, David; Kilburn, Matt R.; Saunders, Martin; et al. (October 2011). "Microfossils of sulphur-metabolizing cells in 3.4-billion-year-old rocks of Western Australia". Nature Geoscience. 4 (10): 698–702. Bibcode:2011NatGe...4..698W. doi:10.1038/ngeo1238.
- ^ Bell, Elizabeth A.; Boehnike, Patrick; Harrison, T. Mark; et al. (19 October 2015). "Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon". PNAS. 112 (47): 14518–14521. Bibcode:2015PNAS..11214518B. doi:10.1073/pnas.1517557112. PMC 4664351. PMID 26483481. Early edition, published online before print.
- ^ Baumgartner, Rafael; Van Kranendonk, Martin; Wacey, David; et al. (2019). "Nano−porous pyrite and organic matter in 3.5-billion-year-old stromatolites record primordial life" (PDF). Geology. 47 (11): 1039–1043. Bibcode:2019Geo....47.1039B. doi:10.1130/G46365.1. S2CID 204258554.
- ^ Djokic, Tara; Van Kranendonk, Martin; Cambell, Kathleen; Walter, Malcolm (2017). "Earliest signs of life on land preserved in ca. 3.5 Ga hot spring deposits". Nature Communications. 3.
- ^ جون ماينارد سميث، إيورز سزاثماري.اصول الحياة: من ولادة الحياة إلى أصل اللغة. مطبعة أوكسفورد. ISBN 0-19-286209-X.
- ^ من مقالة التخلق اللاحيوي من شبكة المجتمع الدولي للتعقيد والمعلومات والتصميم ISCID
- ^ والتر جيلبيرت، (فبراير/شباط 1986) مجلة Nature 319:618
- ^ Landau, Elizabeth (12 October 2016). "Building Blocks of Life's Building Blocks Come From Starlight". NASA. Archived from the original on 13 October 2016. Retrieved 13 October 2016.
- ^ أ ب Ehrenfreund, Pascale; Cami, Jan (December 2010). "Cosmic carbon chemistry: from the interstellar medium to the early Earth". Cold Spring Harbor Perspectives in Biology. 2 (12): a002097. doi:10.1101/cshperspect.a002097. PMC 2982172. PMID 20554702.
- ^ Geballe, Thomas R.; Najarro, Francisco; Figer, Donald F.; et al. (10 November 2011). "Infrared diffuse interstellar bands in the Galactic Centre region". Nature. 479 (7372): 200–202. arXiv:1111.0613. Bibcode:2011Natur.479..200G. doi:10.1038/nature10527. PMID 22048316. S2CID 17223339.
- ^ Klyce 2001
- ^ أ ب ت ث Hoover, Rachel (21 February 2014). "Need to Track Organic Nano-Particles Across the Universe? NASA's Got an App for That". Ames Research Center. NASA. Archived from the original on 6 September 2015. Retrieved 22 June 2015.
- ^ Goncharuk, Vladislav V.; Zui, O. V. (February 2015). "Water and carbon dioxide as the main precursors of organic matter on Earth and in space". Journal of Water Chemistry and Technology. 37 (1): 2–3. doi:10.3103/S1063455X15010026. S2CID 97965067.
- ^ Abou Mrad, Ninette; Vinogradoff, Vassilissa; Duvernay, Fabrice; et al. (2015). "Laboratory experimental simulations: Chemical evolution of the organic matter from interstellar and cometary ice analogs". Bulletin de la Société Royale des Sciences de Liège. 84: 21–32. Bibcode:2015BSRSL..84...21A. Archived from the original on 13 April 2015. Retrieved 6 April 2015.
- ^ Oba, Yasuhiro; et al. (26 April 2022). "Identifying the wide diversity of extraterrestrial purine and pyrimidine nucleobases in carbonaceous meteorites". Nature Communications. 13 (2008): 2008. Bibcode:2022NatCo..13.2008O. doi:10.1038/s41467-022-29612-x. PMC 9042847. PMID 35473908. S2CID 248402205.
- ^ "'Life chemical' detected in comet". BBC News. London. 18 August 2009. Archived from the original on 25 May 2015. Retrieved 23 June 2015.
- ^ Thompson, William Reid; Murray, B. G.; Khare, Bishun Narain; Sagan, Carl (30 December 1987). "Coloration and darkening of methane clathrate and other ices by charged particle irradiation: Applications to the outer solar system". Journal of Geophysical Research. 92 (A13): 14933–14947. Bibcode:1987JGR....9214933T. doi:10.1029/JA092iA13p14933. PMID 11542127.
- ^ Goldman, Nir; Tamblyn, Isaac (20 June 2013). "Prebiotic Chemistry within a Simple Impacting Icy Mixture". Journal of Physical Chemistry A. 117 (24): 5124–5131. Bibcode:2013JPCA..117.5124G. doi:10.1021/jp402976n. PMID 23639050. S2CID 5144843.
- ^ "NASA Ames PAH IR Spectroscopic Database". NASA. Archived from the original on 29 June 2015. Retrieved 17 June 2015.
- ^ أ ب ت Hudgins, Douglas M.; Bauschlicher, Charles W. Jr.; Allamandola, Louis J. (10 October 2005). "Variations in the Peak Position of the 6.2 μm Interstellar Emission Feature: A Tracer of N in the Interstellar Polycyclic Aromatic Hydrocarbon Population". The Astrophysical Journal. 632 (1): 316–332. Bibcode:2005ApJ...632..316H. CiteSeerX 10.1.1.218.8786. doi:10.1086/432495. S2CID 7808613.
- ^ أ ب ت Des Marais, David J.; Allamandola, Louis J.; Sandford, Scott; et al. (2009). "Cosmic Distribution of Chemical Complexity". Ames Research Center. Mountain View, California: NASA. Archived from the original on 27 February 2014. Retrieved 24 June 2015.
- ^ أ ب Carey, Bjorn (18 October 2005). "Life's Building Blocks 'Abundant in Space'". Space.com. Watsonville, California: Imaginova. Archived from the original on 26 June 2015. Retrieved 23 June 2015.
- ^ García-Hernández, Domingo. A.; Manchado, Arturo; García-Lario, Pedro; et al. (20 November 2010). "Formation of Fullerenes in H-Containing Planetary Nebulae". The Astrophysical Journal Letters. 724 (1): L39–L43. arXiv:1009.4357. Bibcode:2010ApJ...724L..39G. doi:10.1088/2041-8205/724/1/L39. S2CID 119121764.
- ^ d'Ischia, Marco; Manini, Paola; Moracci, Marco; et al. (21 August 2019). "Astrochemistry and Astrobiology: Materials Science in Wonderland?". International Journal of Molecular Sciences. 20 (17): 4079. doi:10.3390/ijms20174079. PMC 6747172. PMID 31438518.
- ^ Gudipati, Murthy S.; Yang, Rui (1 September 2012). "In-situ Probing of Radiation-induced Processing of Organics in Astrophysical Ice Analogs – Novel Laser Desorption Laser Ionization Time-of-flight Mass Spectroscopic Studies". The Astrophysical Journal Letters. 756 (1): L24. Bibcode:2012ApJ...756L..24G. doi:10.1088/2041-8205/756/1/L24. S2CID 5541727.
- ^ أ ب Gallori, Enzo (June 2011). "Astrochemistry and the origin of genetic material". Rendiconti Lincei. 22 (2): 113–118. doi:10.1007/s12210-011-0118-4. S2CID 96659714. "Paper presented at the Symposium 'Astrochemistry: molecules in space and time' (Rome, 4–5 November 2010), sponsored by Fondazione 'Guido Donegani', Accademia Nazionale dei Lincei."
- ^ Martins, Zita (February 2011). "Organic Chemistry of Carbonaceous Meteorites". Elements. 7 (1): 35–40. doi:10.2113/gselements.7.1.35.
- ^ Martins, Zita; Botta, Oliver; Fogel, Marilyn L.; et al. (15 June 2008). "Extraterrestrial nucleobases in the Murchison meteorite". Earth and Planetary Science Letters. 270 (1–2): 130–136. arXiv:0806.2286. Bibcode:2008E&PSL.270..130M. doi:10.1016/j.epsl.2008.03.026. S2CID 14309508.
- ^ Callahan, Michael P.; Smith, Karen E.; Cleaves, H. James, II; et al. (23 August 2011). "Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases". PNAS. 108 (34): 13995–13998. Bibcode:2011PNAS..10813995C. doi:10.1073/pnas.1106493108. PMC 3161613. PMID 21836052.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Steigerwald, John (8 August 2011). "NASA Researchers: DNA Building Blocks Can Be Made in Space". Goddard Space Flight Center. NASA. Archived from the original on 23 June 2015. Retrieved 23 June 2015.
- ^ Kwok, Sun; Zhang, Yong (3 November 2011). "Mixed aromatic–aliphatic organic nanoparticles as carriers of unidentified infrared emission features". Nature. 479 (7371): 80–83. Bibcode:2011Natur.479...80K. doi:10.1038/nature10542. PMID 22031328. S2CID 4419859.
- ^ Jørgensen, Jes K.; Favre, Cécile; Bisschop, Suzanne E.; et al. (2012). "Detection of the simplest sugar, glycolaldehyde, in a solar-type protostar with ALMA" (PDF). The Astrophysical Journal Letters. 757 (1): L4. arXiv:1208.5498. Bibcode:2012ApJ...757L...4J. doi:10.1088/2041-8205/757/1/L4. S2CID 14205612. Archived (PDF) from the original on 24 September 2015. Retrieved 2015-06-23.
- ^ Furukawa, Yoshihiro; Chikaraishi, Yoshito; Ohkouchi, Naohiko; et al. (2019-11-13). "Extraterrestrial ribose and other sugars in primitive meteorites". PNAS. 116 (49): 24440–24445. Bibcode:2019PNAS..11624440F. doi:10.1073/pnas.1907169116. PMC 6900709. PMID 31740594.
- ^ Oró, Joan; Kimball, Aubrey P. (February 1962). "Synthesis of purines under possible primitive earth conditions: II. Purine intermediates from hydrogen cyanide". Archives of Biochemistry and Biophysics. 96 (2): 293–313. doi:10.1016/0003-9861(62)90412-5. PMID 14482339.
- ^ Cleaves II, Henderson (2010). "The origin of the biologically coded amino acids". Journal of Theoretical Biology. 263 (4): 490–498. Bibcode:2010JThBi.263..490C. doi:10.1016/j.jtbi.2009.12.014. PMID 20034500.
- ^ Breslow, R. (1959). "On the Mechanism of the Formose Reaction". Tetrahedron Letters. 1 (21): 22–26. doi:10.1016/S0040-4039(01)99487-0.
- ^ Oró, Joan (16 September 1961). "Mechanism of Synthesis of Adenine from Hydrogen Cyanide under Possible Primitive Earth Conditions". Nature. 191 (4794): 1193–1194. Bibcode:1961Natur.191.1193O. doi:10.1038/1911193a0. PMID 13731264. S2CID 4276712.
- ^ أ ب Saladino, Raffaele; Crestini, Claudia; Pino, Samanta; et al. (March 2012). "Formamide and the origin of life" (PDF). Physics of Life Reviews. 9 (1): 84–104. Bibcode:2012PhLRv...9...84S. doi:10.1016/j.plrev.2011.12.002. hdl:2108/85168. PMID 22196896.
- ^ أ ب Saladino, Raffaele; Botta, Giorgia; Pino, Samanta; et al. (July 2012). "From the one-carbon amide formamide to RNA all the steps are prebiotically possible". Biochimie. 94 (7): 1451–1456. doi:10.1016/j.biochi.2012.02.018. hdl:11573/515604. PMID 22738728.
- ^ Marlaire, Ruth, ed. (3 March 2015). "NASA Ames Reproduces the Building Blocks of Life in Laboratory". Ames Research Center. NASA. Archived from the original on 5 March 2015. Retrieved 5 March 2015.
- ^ Ferus, Martin; Nesvorný, David; Šponer, Jiří; et al. (2015). "High-energy chemistry of formamide: A unified mechanism of nucleobase formation". PNAS. 112 (3): 657–662. Bibcode:2015PNAS..112..657F. doi:10.1073/pnas.1412072111. PMC 4311869. PMID 25489115.
- ^ Basile, Brenda; Lazcano, Antonio; Oró, Joan (1984). "Prebiotic syntheses of purines and pyrimidines". Advances in Space Research. 4 (12): 125–131. Bibcode:1984AdSpR...4l.125B. doi:10.1016/0273-1177(84)90554-4. PMID 11537766.
- ^ Orgel, Leslie E. (August 2004). "Prebiotic Adenine Revisited: Eutectics and Photochemistry". Origins of Life and Evolution of Biospheres. 34 (4): 361–369. Bibcode:2004OLEB...34..361O. doi:10.1023/B:ORIG.0000029882.52156.c2. PMID 15279171. S2CID 4998122.
- ^ Robertson, Michael P.; Miller, Stanley L. (29 June 1995). "An efficient prebiotic synthesis of cytosine and uracil". Nature. 375 (6534): 772–774. Bibcode:1995Natur.375..772R. doi:10.1038/375772a0. PMID 7596408. S2CID 4351012.
- ^ Fox, Douglas (February 2008). "Did Life Evolve in Ice?". Discover. Archived from the original on 30 June 2008. Retrieved 2008-07-03.
- ^ Levy, Matthew; Miller, Stanley L.; Brinton, Karen; Bada, Jeffrey L. (June 2000). "Prebiotic Synthesis of Adenine and Amino Acids Under Europa-like Conditions". Icarus. 145 (2): 609–613. Bibcode:2000Icar..145..609L. doi:10.1006/icar.2000.6365. PMID 11543508.
- ^ Menor-Salván, César; Ruiz-Bermejo, Marta; Guzmán, Marcelo I.; et al. (20 April 2009). "Synthesis of Pyrimidines and Triazines in Ice: Implications for the Prebiotic Chemistry of Nucleobases". Chemistry: A European Journal. 15 (17): 4411–4418. doi:10.1002/chem.200802656. PMID 19288488.
- ^ Roy, Debjani; Najafian, Katayoun; von Ragué Schleyer, Paul (30 October 2007). "Chemical evolution: The mechanism of the formation of adenine under prebiotic conditions". PNAS. 104 (44): 17272–17277. Bibcode:2007PNAS..10417272R. doi:10.1073/pnas.0708434104. PMC 2077245. PMID 17951429.
- ^ Lancet, Doron (30 December 2014). "Systems Prebiology-Studies of the origin of Life". The Lancet Lab. Rehovot, Israel: Department of Molecular Genetics; Weizmann Institute of Science. Archived from the original on 26 June 2015. Retrieved 26 June 2015.
- ^ Segré, Daniel; Ben-Eli, Dafna; Deamer, David W.; Lancet, Doron (February 2001). "The Lipid World" (PDF). Origins of Life and Evolution of Biospheres. 31 (1–2): 119–145. Bibcode:2001OLEB...31..119S. doi:10.1023/A:1006746807104. PMID 11296516. S2CID 10959497. Archived (PDF) from the original on 26 June 2015.
- ^ أ ب ت Chen, Irene A.; Walde, Peter (July 2010). "From Self-Assembled Vesicles to Protocells". Cold Spring Harbor Perspectives in Biology. 2 (7): a002170. doi:10.1101/cshperspect.a002170. PMC 2890201. PMID 20519344.
- ^ Eigen, Manfred; Schuster, Peter (November 1977). "The Hypercycle. A Principle of Natural Self-Organization. Part A: Emergence of the Hypercycle" (PDF). Naturwissenschaften. 64 (11): 541–65. Bibcode:1977NW.....64..541E. doi:10.1007/bf00450633. PMID 593400. S2CID 42131267. Archived from the original (PDF) on 3 March 2016.
- Eigen, Manfred; Schuster, Peter (1978). "The Hypercycle. A Principle of Natural Self-Organization. Part B: The Abstract Hypercycle" (PDF). Naturwissenschaften. 65 (1): 7–41. Bibcode:1978NW.....65....7E. doi:10.1007/bf00420631. S2CID 1812273. Archived from the original (PDF) on 3 March 2016.
- Eigen, Manfred; Schuster, Peter (July 1978). "The Hypercycle. A Principle of Natural Self-Organization. Part C: The Realistic Hypercycle" (PDF). Naturwissenschaften. 65 (7): 341–369. Bibcode:1978NW.....65..341E. doi:10.1007/bf00439699. S2CID 13825356. Archived from the original (PDF) on 16 June 2016.
- ^ Markovitch, Omer; Lancet, Doron (Summer 2012). "Excess Mutual Catalysis Is Required for Effective Evolvability". Artificial Life. 18 (3): 243–266. doi:10.1162/artl_a_00064. PMID 22662913. S2CID 5236043.
- ^ Tessera, Marc (2011). "Origin of Evolution versus Origin of Life: A Shift of Paradigm". International Journal of Molecular Sciences. 12 (6): 3445–3458. doi:10.3390/ijms12063445. PMC 3131571. PMID 21747687. Special Issue: "Origin of Life 2011"
- ^ Onsager, Lars (1931). "Reciprocal Relations in Irreversible Processes I and II". Physical Review. 37 (4): 405. Bibcode:1931PhRv...37..405O. doi:10.1103/PhysRev.37.405.
- ^ Onsager, Lars (1931). "Reciprocal Relations in Irreversible Processes I and II". Physical Review (38): 2265. doi:10.1103/PhysRev.38.2265.
- ^ Prigogine, Ilya (1967). An Introduction to the Thermodynamics of Irreversible Processes. New York: Wiley.
- ^ "Exploring Life's Origins: Protocells". Exploring Life's Origins: A Virtual Exhibit. Arlington County, Virginia: National Science Foundation. Archived from the original on 28 February 2014. Retrieved 18 March 2014.
- ^ أ ب ت Chen, Irene A. (8 December 2006). "The Emergence of Cells During the Origin of Life". Science. 314 (5805): 1558–1559. doi:10.1126/science.1137541. PMID 17158315.
- ^ Zimmer, Carl (26 June 2004). "What Came Before DNA?". Discover. Archived from the original on 19 March 2014.
- ^ Shapiro, Robert (June 2007). "A Simpler Origin for Life". Scientific American. 296 (6): 46–53. Bibcode:2007SciAm.296f..46S. doi:10.1038/scientificamerican0607-46. PMID 17663224. Archived from the original on 14 June 2015.
- ^ Chang 2007
- ^ أ ب ت ث Lane, Nick (2015). The Vital Question: Why Is Life The Way It Is?. Profile Books. pp. 129–140. ISBN 978-1781250365.
- ^ Sharov, Alexei A.; Gordon, Richard (2018). "Life Before Earth". Habitability of the Universe Before Earth: Life Before Earth. Astrobiology Exploring Life on Earth and Beyond. Academic Press. pp. 265–296. doi:10.1016/B978-0-12-811940-2.00011-3. ISBN 9780128119402. S2CID 117048600.
- ^ Ladyman, J.; Lambert, J.; Weisner, K. B. (2013). "What is a Complex System?". European Journal of the Philosophy of Science. 3: 33–67. doi:10.1007/s13194-012-0056-8. S2CID 18787276.
- ^ Esposito, M.; Lindenberg, Katja; Van den Broeck, C. (2010). "Entropy production as correlation between system and reservoir". New Journal of Physics. 12 (1): 013013. arXiv:0908.1125. Bibcode:2010NJPh...12a3013E. doi:10.1088/1367-2630/12/1/013013. S2CID 26657293.
- ^ Bar-Nun, A.; Bar-Nun, N.; Bauer, S. H.; Sagan, Carl (24 April 1970). "Shock Synthesis of Amino Acids in Simulated Primitive Environments". Science. 168 (3930): 470–473. Bibcode:1970Sci...168..470B. doi:10.1126/science.168.3930.470. PMID 5436082. S2CID 42467812.
- ^ Anbar, Michael (27 September 1968). "Cavitation during Impact of Liquid Water on Water: Geochemical Implications". Science. 161 (3848): 1343–1344. Bibcode:1968Sci...161.1343A. doi:10.1126/science.161.3848.1343. PMID 17831346.
- ^ Dharmarathne, Leena; Grieser, Franz (7 January 2016). "Formation of Amino Acids on the Sonolysis of Aqueous Solutions Containing Acetic Acid, Methane, or Carbon Dioxide, in the Presence of Nitrogen Gas". The Journal of Physical Chemistry A. 120 (2): 191–199. Bibcode:2016JPCA..120..191D. doi:10.1021/acs.jpca.5b11858. PMID 26695890.
- ^ Patehebieke, Yeersen; Zhao, Ze-Run; Wang, Su; Xu, Hao-Xing; Chen, Qian-Qian; Wang, Xiao (2021). "Cavitation as a plausible driving force for the prebiotic formation of N9 purine nucleosides". Cell Reports Physical Science. 2 (3): 100375. Bibcode:2021CRPS....200375P. doi:10.1016/j.xcrp.2021.100375. S2CID 233662126.
- ^ Kalson, Natan-Haim; Furman, David; Zeiri, Yehuda (11 September 2017). "Cavitation-Induced Synthesis of Biogenic Molecules on Primordial Earth". ACS Central Science. 3 (9): 1041–1049. doi:10.1021/acscentsci.7b00325. PMC 5620973. PMID 28979946. S2CID 21409351.
- ^ Muller, Anthonie W. J. (1995). "Were the first organisms heat engines? A new model for biogenesis and the early evolution of biological energy conversion". Progress in Biophysics and Molecular Biology. 63 (2): 193–231. doi:10.1016/0079-6107(95)00004-7. PMID 7542789.
- ^ Muller, Anthonie W. J.; Schulze-Makuch, Dirk (2006). "Thermal energy and the origin of life". Origins of Life and Evolution of Biospheres. 36 (2): 77–189. Bibcode:2006OLEB...36..177M. doi:10.1007/s11084-005-9003-4. PMID 16642267. S2CID 22179552.
- ^ Junge, Wolfgang; Nelson, Nathan (2 June 2015). "ATP Synthase". Annual Review of Biochemistry. 84 (1): 631–657. doi:10.1146/annurev-biochem-060614-034124. PMID 25839341.
- ^ Benner, S. A.; Bell, E. A.; Biondi, E.; Brasser, R.; Carell, T.; Kim, H.-J.; Mojzsis, S. J.; Omran, A.; Pasek, M. A.; Trail, D. (2020). "When Did Life Likely Emerge on Earth in an RNA-First Process?". ChemSystemsChem. 2 (2). doi:10.1002/syst.201900035.
- ^ * Copley, Shelley D.; Smith, Eric; Morowitz, Harold J. (December 2007). "The origin of the RNA world: Co-evolution of genes and metabolism" (PDF). Bioorganic Chemistry. 35 (6): 430–443. doi:10.1016/j.bioorg.2007.08.001. PMID 17897696. Archived (PDF) from the original on 5 September 2013. Retrieved 8 June 2015.
The proposal that life on Earth arose from an RNA world is widely accepted.
- Orgel, Leslie E. (April 2003). "Some consequences of the RNA world hypothesis". Origins of Life and Evolution of Biospheres. 33 (2): 211–218. Bibcode:2003OLEB...33..211O. doi:10.1023/A:1024616317965. PMID 12967268. S2CID 32779859.
It now seems very likely that our familiar DNA/RNA/protein world was preceded by an RNA world...
- Robertson & Joyce 2012: "There is now strong evidence indicating that an RNA World did indeed exist before DNA- and protein-based life."
- Neveu, Kim & Benner 2013: "[The RNA world's existence] has broad support within the community today."
- Orgel, Leslie E. (April 2003). "Some consequences of the RNA world hypothesis". Origins of Life and Evolution of Biospheres. 33 (2): 211–218. Bibcode:2003OLEB...33..211O. doi:10.1023/A:1024616317965. PMID 12967268. S2CID 32779859.
- ^ أ ب ت Robertson, Michael P.; Joyce, Gerald F. (May 2012). "The origins of the RNA world". Cold Spring Harbor Perspectives in Biology. 4 (5): a003608. doi:10.1101/cshperspect.a003608. PMC 3331698. PMID 20739415.
- ^ أ ب ت Cech, Thomas R. (July 2012). "The RNA Worlds in Context". Cold Spring Harbor Perspectives in Biology. 4 (7): a006742. doi:10.1101/cshperspect.a006742. PMC 3385955. PMID 21441585.
- ^ Pearce, Ben K. D.; Pudritz, Ralph E.; Semenov, Dmitry A.; Henning, Thomas K. (24 October 2017). "Origin of the RNA world: The fate of nucleobases in warm little ponds". PNAS. 114 (43): 11327–11332. arXiv:1710.00434. Bibcode:2017PNAS..11411327P. doi:10.1073/pnas.1710339114. PMC 5664528. PMID 28973920.
- ^ Yarus, Michael (April 2011). "Getting Past the RNA World: The Initial Darwinian Ancestor". Cold Spring Harbor Perspectives in Biology. 3 (4): a003590. doi:10.1101/cshperspect.a003590. PMC 3062219. PMID 20719875.
- ^ Voet & Voet 2004, p. 29
- ^ Fox, George.E. (9 June 2010). "Origin and evolution of the ribosome". Cold Spring Harbor Perspectives in Biology. 2 (9(a003483)): a003483. doi:10.1101/cshperspect.a003483. PMC 2926754. PMID 20534711.
- ^ Neveu, Marc; Kim, Hyo-Joong; Benner, Steven A. (22 April 2013). "The 'Strong' RNA World Hypothesis: Fifty Years Old". Astrobiology. 13 (4): 391–403. Bibcode:2013AsBio..13..391N. doi:10.1089/ast.2012.0868. PMID 23551238.
- ^ Gilbert, Walter (20 February 1986). "Origin of life: The RNA world". Nature. 319 (6055): 618. Bibcode:1986Natur.319..618G. doi:10.1038/319618a0. S2CID 8026658.
- ^ Orgel, Leslie E. (October 1994). "The origin of life on Earth". Scientific American. 271 (4): 76–83. Bibcode:1994SciAm.271d..76O. doi:10.1038/scientificamerican1094-76. PMID 7524147.
- ^ Lincoln, Tracey A.; Joyce, Gerald F. (27 February 2009). "Self-Sustained Replication of an RNA Enzyme". Science. 323 (5918): 1229–1232. Bibcode:2009Sci...323.1229L. doi:10.1126/science.1167856. PMC 2652413. PMID 19131595.
- ^ Joyce, Gerald F. (2009). "Evolution in an RNA world". Cold Spring Harbor Perspectives in Biology. 74 (Evolution: The Molecular Landscape): 17–23. doi:10.1101/sqb.2009.74.004. PMC 2891321. PMID 19667013.
- ^ Szostak, Jack W. (5 February 2015). "The Origins of Function in Biological Nucleic Acids, Proteins, and Membranes". Chevy Chase, Maryland: Howard Hughes Medical Institute. Archived from the original on 14 July 2015. Retrieved 16 June 2015.
- ^ Bernstein, Harris; Byerly, Henry C.; Hopf, Frederick A.; et al. (June 1983). "The Darwinian Dynamic". The Quarterly Review of Biology. 58 (2): 185–207. doi:10.1086/413216. JSTOR 2828805. S2CID 83956410.
- ^ Michod 1999
- ^ Palasek, Stan (23 May 2013). "Primordial RNA Replication and Applications in PCR Technology". arXiv:1305.5581v1 [q-bio.BM].
- ^ Vlassov, Alexander V.; Kazakov, Sergei A.; Johnston, Brian H.; et al. (August 2005). "The RNA World on Ice: A New Scenario for the Emergence of RNA Information". Journal of Molecular Evolution. 61 (2): 264–273. Bibcode:2005JMolE..61..264V. doi:10.1007/s00239-004-0362-7. PMID 16044244. S2CID 21096886.
- ^ Nussinov, Mark D.; Otroshchenko, Vladimir A.; Santoli, Salvatore (1997). "The emergence of the non-cellular phase of life on the fine-grained clayish particles of the early Earth's regolith". BioSystems. 42 (2–3): 111–118. doi:10.1016/S0303-2647(96)01699-1. PMID 9184757.
- ^ Kühnlein, Alexandra; Lanzmich, Simon A.; Brun, Dieter (2 March 2021). "tRNA sequences can assemble into a replicator". eLife. 10. doi:10.7554/eLife.63431. PMC 7924937. PMID 33648631.
- ^ Noller, Harry F. (April 2012). "Evolution of protein synthesis from an RNA world". Cold Spring Harbor Perspectives in Biology. 4 (4): a003681. doi:10.1101/cshperspect.a003681. PMC 3312679. PMID 20610545.
- ^ Koonin, Eugene V. (31 May 2007). "The cosmological model of eternal inflation and the transition from chance to biological evolution in the history of life". Biology Direct. 2: 15. doi:10.1186/1745-6150-2-15. PMC 1892545. PMID 17540027.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Boone, David R.; Castenholz, Richard W.; Garrity, George M., eds. (2001). The Archaea and the Deeply Branching and Phototrophic Bacteria. Bergey's Manual of Systematic Bacteriology. Springer. ISBN 978-0-387-21609-6. Archived from the original on 25 December 2014.[صفحة مطلوبة]
- ^ Woese, C. R.; Fox, G. E. (1977). "Phylogenetic structure of the prokaryotic domain: the primary kingdoms". PNAS. 7 (11): 5088–5090. Bibcode:1977PNAS...74.5088W. doi:10.1073/pnas.74.11.5088. PMC 432104. PMID 270744.
- ^ Valas, R. E.; Bourne, P. E. (2011). "The origin of a derived superkingdom: how a gram-positive bacterium crossed the desert to become an archaeon". Biology Direct. 6: 16. doi:10.1186/1745-6150-6-16. PMC 3056875. PMID 21356104.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Cavalier-Smith, Thomas (2006). "Rooting the tree of life by transition analyses". Biology Direct. 1: 19. doi:10.1186/1745-6150-1-19. PMC 1586193. PMID 16834776.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Early life liked it hot". Nature. 535 (7613): 468. 2016. doi:10.1038/535468b. S2CID 49905802.
- ^ Gogarten, Johann Peter; Deamer, David (2016-11-25). "Is LUCA a thermophilic progenote?". Nature Microbiology (in الإنجليزية). 1 (12): 1–2. doi:10.1038/nmicrobiol.2016.229. ISSN 2058-5276.
- ^ Catchpole, Ryan; Forterre, Patrick (2019). "The evolution of Reverse Gyrase suggests a non-hyperthermophilic Last Universal Common Ancestor". Molecular Biology and Evolution. doi:10.1093/molbev/msz180.
- ^ Berkemer, Sarah J.; McGlynn, Shawn E (August 8, 2020). "A New Analysis of Archaea–Bacteria Domain Separation: Variable Phylogenetic Distance and the Tempo of Early Evolution". Molecular Biology and Evolution. 37 (8): 2332–2340. doi:10.1093/molbev/msaa089. PMC 7403611. PMID 32316034.
- ^ Hoffmann, Geoffrey W. (25 June 1974). "On the origin of the genetic code and the stability of the translation apparatus". Journal of Molecular Biology. 86 (2): 349–362. doi:10.1016/0022-2836(74)90024-2. PMID 4414916.
- ^ Orgel, Leslie E. (April 1963). "The Maintenance of the Accuracy of Protein Synthesis and its Relevance to Ageing". PNAS. 49 (4): 517–521. Bibcode:1963PNAS...49..517O. doi:10.1073/pnas.49.4.517. PMC 299893. PMID 13940312.
- ^ Hoffmann, Geoffrey W. (October 1975). "The Stochastic Theory of the Origin of the Genetic Code". Annual Review of Physical Chemistry. 26: 123–144. Bibcode:1975ARPC...26..123H. doi:10.1146/annurev.pc.26.100175.001011.
- ^ Brasier, M. D. (2012). Secret Chambers: The Inside Story of Cells and Complex Life. Oxford University Press. p. 298.
- ^ Ward, Peter & Kirschvink, Joe, op cit, p. 42
- ^ أ ب Colín-García, M.; Heredia, A.; Cordero, G.; et al. (2016). "Hydrothermal vents and prebiotic chemistry: a review". Boletín de la Sociedad Geológica Mexicana. 68 (3): 599–620. doi:10.18268/BSGM2016v68n3a13. Archived from the original on 18 August 2017.
- ^ Schirber, Michael (24 June 2014). "Hydrothermal Vents Could Explain Chemical Precursors to Life". NASA Astrobiology: Life in the Universe. NASA. Archived from the original on 29 November 2014. Retrieved 19 June 2015.
- ^ أ ب Martin, William; Russell, Michael J. (29 January 2003). "On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells". Philosophical Transactions of the Royal Society B. 358 (1429): 59–83, discussion 83–85. doi:10.1098/rstb.2002.1183. PMC 1693102. PMID 12594918.
- ^ أ ب Lane 2009
- ^ Usher, Oli (27 April 2015). "Chemistry of seabed's hot vents could explain emergence of life" (Press release). University College London. Archived from the original on 20 June 2015. Retrieved 19 June 2015.
- ^ Roldan, Alberto; Hollingsworth, Nathan; Roffey, Anna; et al. (May 2015). "Bio-inspired CO2 conversion by iron sulfide catalysts under sustainable conditions". Chemical Communications. 51 (35): 7501–7504. doi:10.1039/C5CC02078F. PMID 25835242. Archived from the original on 20 June 2015. Retrieved 2015-06-19.
- ^ Baross, J. A.; Hoffman, S. E. (1985). "Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life". Origins of Life and Evolution of Biospheres. 15 (4): 327–345. Bibcode:1985OrLi...15..327B. doi:10.1007/bf01808177. S2CID 4613918.
- ^ Russell, M. J.; Hall, A. J. (1997). "The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front". Journal of the Geological Society. 154 (3): 377–402. Bibcode:1997JGSoc.154..377R. doi:10.1144/gsjgs.154.3.0377. PMID 11541234. S2CID 24792282.
- ^ Amend, J. P.; LaRowe, D. E.; McCollom, T. M.; Shock, E. L. (2013). "The energetics of organic synthesis inside and outside the cell". Philosophical Transactions of the Royal Society B. 368 (1622): 20120255. doi:10.1098/rstb.2012.0255. PMC 3685458. PMID 23754809.
- ^ Shock, E. L.; Boyd, E. S. (2015). "Geomicrobiology and microbial geochemistry:principles of geobiochemistry". Elements. 11: 389–394. doi:10.2113/gselements.11.6.395.
- ^ Martin, W.; Russell, M. J. (2007). "On the origin of biochemistry at an alkaline hydrothermal vent". Philosophical Transactions of the Royal Society B. 362 (1486): 1887–1925. doi:10.1098/rstb.2006.1881. PMC 2442388. PMID 17255002.
- ^ Weiss, Madeline C.; Sousa, Filipa L.; Mrnjavac, Natalia; Neukirchen, Sinje; Roettger, Mayo; Nelson-Sathi, Shijulal; Martin, William F. (2016-07-25). "The physiology and habitat of the last universal common ancestor" (PDF). Nature Microbiology. 1 (9): 16116. doi:10.1038/nmicrobiol.2016.116. PMID 27562259. S2CID 2997255.
- ^ Colín-García, María (2016). "Hydrothermal vents and prebiotic chemistry: a review". Boletín de la Sociedad Geológica Mexicana. 68 (3): 599–620. doi:10.18268/BSGM2016v68n3a13. Archived from the original on August 18, 2017.
- ^ Mulkidjanian, Armen Y.; Bychkov, Andrew Yu.; Dibrova, Daria V.; Galperin, Michael Y.; Koonin, Eugene V. (2012-04-03). "Origin of first cells at terrestrial, anoxic geothermal fields". Proceedings of the National Academy of Sciences (in الإنجليزية). 109 (14). doi:10.1073/pnas.1117774109. ISSN 0027-8424. PMC 3325685. PMID 22331915.
- ^ Chandru, Kuhan; Guttenberg, Nicholas; Giri, Chaitanya; et al. (31 May 2018). "Simple prebiotic synthesis of high diversity dynamic combinatorial polyester libraries". Communications Chemistry. 1 (1). doi:10.1038/s42004-018-0031-1.
- ^ Forsythe, Jay G.; Yu, Sheng-Sheng; Mamajanov, Irena; et al. (17 August 2015). "Ester-Mediated Amide Bond Formation Driven by Wet–Dry Cycles: A Possible Path to Polypeptides on the Prebiotic Earth". Angewandte Chemie International Edition in English. 54 (34): 9871–9875. doi:10.1002/anie.201503792. PMC 4678426. PMID 26201989.
- ^ Patel, Bhavesh H.; Percivalle, Claudia; Ritson, Dougal J.; Duffy, Colm. D.; Sutherland, John D. (March 16, 2015). "Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism". Nature chemistry. 7 (4): 301–307. doi:10.1038/nchem.2202. ISSN 1755-4330. PMC 4568310. PMID 25803468.
- ^ Mulkidjanian, Armid; Bychkov, Andrew; Dibrova, Daria; Galperin, Michael; Koonin, Eugene (3 April 2012). "Origin of first cells at terrestrial, anoxic geothermal fields". PNAS. 109 (14): E821–E830. Bibcode:2012PNAS..109E.821M. doi:10.1073/pnas.1117774109. PMC 3325685. PMID 22331915.
- ^ Damer, Bruce; Deamer, David (1 April 2020). "The Hot Spring Hypothesis for an Origin of Life". Astrobiology. 20 (4): 429–452. Bibcode:2020AsBio..20..429D. doi:10.1089/ast.2019.2045. PMC 7133448. PMID 31841362.
- ^ Deamer, David (10 February 2021). "Where Did Life Begin? Testing Ideas in Prebiotic Analogue Conditions". Life. 11 (2): 134. doi:10.3390/life11020134.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Cairns-Smith, Graham (2 September 1982). Genetic Takeover and the Mineral Origins of Life. Cambridge: Cambridge University Press. ISBN 0-521-23312-7. OCLC 7875600.
- ^ Dawkins 1996, pp. 148–161
- ^ Huang, Wenhua; Ferris, James P. (12 July 2006). "One-Step, Regioselective Synthesis of up to 50-mers of RNA Oligomers by Montmorillonite Catalysis". Journal of the American Chemical Society. 128 (27): 8914–8919. doi:10.1021/ja061782k. PMID 16819887.
- ^ Subramaniam, Anand Bala; Wan, Jiandi; Gopinath, Arvind; Stone, Howard A. (2011). "Semi-permeable vesicles composed of natural clay" (PDF). Soft Matter. 7 (6): 2600–2612. arXiv:1011.4711. Bibcode:2011SMat....7.2600S. doi:10.1039/c0sm01354d. S2CID 52253528.
- ^ Hartman, Hyman (1998). "Photosynthesis and the Origin of Life". Origins of Life and Evolution of Biospheres. 28 (4–6): 515–521. Bibcode:1998OLEB...28..515H. doi:10.1023/A:1006548904157. PMID 11536891. S2CID 2464.
- ^ Yue-Ching Ho, Eugene (July–September 1990). "Evolutionary Epistemology and Sir Karl Popper's Latest Intellectual Interest: A First-Hand Report". Intellectus. 15: 1–3. OCLC 26878740. Archived from the original on 11 March 2012.
- ^ Popper, Karl R. (29 March 1990). "Pyrite and the origin of life". Nature. 344 (6265): 387. Bibcode:1990Natur.344..387P. doi:10.1038/344387a0. S2CID 4322774.
- ^ أ ب Keller, Markus A.; Turchyn, Alexandra V.; Ralser, Markus (25 March 2014). "Non-enzymatic glycolysis and pentose phosphate pathway-like reactions in a plausible Archean ocean". Molecular Systems Biology. 10 (725): 725. doi:10.1002/msb.20145228. PMC 4023395. PMID 24771084.
- ^ Huber, Claudia; Wächtershäuser, Günter (31 July 1998). "Peptides by Activation of Amino Acids with CO on (Ni,Fe)S Surfaces: Implications for the Origin of Life". Science. 281 (5377): 670–672. Bibcode:1998Sci...281..670H. doi:10.1126/science.281.5377.670. PMID 9685253.
- ^ Adamala, Katarzyna; Szostak, Jack W. (29 November 2013). "Nonenzymatic Template-Directed RNA Synthesis Inside Model Protocells". Science. 342 (6162): 1098–1100. Bibcode:2013Sci...342.1098A. doi:10.1126/science.1241888. PMC 4104020. PMID 24288333.
- ^ Musser, George (23 September 2011). "How Life Arose on Earth, and How a Singularity Might Bring It Down". Observations (Blog). Archived from the original on 17 June 2015. Retrieved 17 June 2015.
- ^ Carroll, Sean (10 March 2010). "Free Energy and the Meaning of Life". Cosmic Variance (Blog). Discover. Archived from the original on 14 July 2015. Retrieved 17 June 2015.
- ^ England, Jeremy L. (28 September 2013). "Statistical physics of self-replication" (PDF). Journal of Chemical Physics. 139 (12): 121923. arXiv:1209.1179. Bibcode:2013JChPh.139l1923E. doi:10.1063/1.4818538. hdl:1721.1/90392. PMID 24089735. S2CID 478964. Archived (PDF) from the original on 4 June 2015.
- ^ Fox, Ronald F. (December 1993). "Review of Stuart Kauffman, The Origins of Order: Self-Organization and Selection in Evolution". Biophysical Journal. 65 (6): 2698–2699. Bibcode:1993BpJ....65.2698F. doi:10.1016/s0006-3495(93)81321-3. PMC 1226010.
- ^ Orgel, Leslie E. (7 November 2000). "Self-organizing biochemical cycles". PNAS. 97 (23): 12503–12507. Bibcode:2000PNAS...9712503O. doi:10.1073/pnas.220406697. PMC 18793. PMID 11058157.
- ^ Chandru, Kuhan; Gilbert, Alexis; Butch, Christopher; Aono, Masashi; Cleaves, Henderson James II (21 July 2016). "The Abiotic Chemistry of Thiolated Acetate Derivatives and the Origin of Life". Scientific Reports. 6 (29883): 29883. Bibcode:2016NatSR...629883C. doi:10.1038/srep29883. PMC 4956751. PMID 27443234.
- ^ Vallee, Yannick; Shalayel, Ibrahim; Ly, Kieu-Dung; Rao, K. V. Raghavendra; Paëpe, Gael De; Märker, Katharina; Milet, Anne (8 November 2017). "At the very beginning of life on Earth: the thiol-rich peptide (TRP) world hypothesis". The International Journal of Developmental Biology. 61 (8–9): 471–478. doi:10.1387/ijdb.170028yv. PMID 29139533.
- ^ أ ب Mulkidjanian, Armen Y. (24 August 2009). "On the origin of life in the zinc world: 1. Photosynthesizing, porous edifices built of hydrothermally precipitated zinc sulfide as cradles of life on Earth". Biology Direct. 4: 26. doi:10.1186/1745-6150-4-26. PMC 3152778. PMID 19703272.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Wächtershäuser, Günter (December 1988). "Before Enzymes and Templates: Theory of Surface Metabolism". Microbiological Reviews. 52 (4): 452–484. doi:10.1128/MMBR.52.4.452-484.1988. PMC 373159. PMID 3070320.
- ^ Mulkidjanian, Armen Y.; Galperin, Michael Y. (24 August 2009). "On the origin of life in the zinc world. 2. Validation of the hypothesis on the photosynthesizing zinc sulfide edifices as cradles of life on Earth". Biology Direct. 4: 27. doi:10.1186/1745-6150-4-27. PMC 2749021. PMID 19703275.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Macallum, A. B. (1 April 1926). "The Paleochemistry of the body fluids and tissues". Physiological Reviews. 6 (2): 316–357. doi:10.1152/physrev.1926.6.2.316.
- ^ Mulkidjanian, Armen Y.; Bychkov, Andrew Yu.; Dibrova, Daria V.; et al. (3 April 2012). "Origin of first cells at terrestrial, anoxic geothermal fields". PNAS. 109 (14): E821–E830. Bibcode:2012PNAS..109E.821M. doi:10.1073/pnas.1117774109. PMC 3325685. PMID 22331915.
- ^ See also Lankenau 2011, pp. 225–286, interconnecting the "Two RNA worlds" concept, and Davidovich, Chen; Belousoff, Matthew; Bashan, Anat; Yonath, Ada (September 2009). "The evolving ribosome: from non-coded peptide bond formation to sophisticated translation machinery". Research in Microbiology. 160 (7): 487–492. doi:10.1016/j.resmic.2009.07.004. PMID 19619641.
- ^ أ ب Plasson, Raphaël; Kondepudi, Dilip K.; Bersini, Hugues; et al. (August 2007). "Emergence of homochirality in far-from-equilibrium systems: Mechanisms and role in prebiotic chemistry". Chirality. 19 (8): 589–600. doi:10.1002/chir.20440. PMID 17559107. "Special Issue: Proceedings from the Eighteenth International Symposium on Chirality (ISCD-18), Busan, Korea, 2006"
- ^ Chaichian, Rojas & Tureanu 2014, pp. 353–364
- ^ Jafarpour, Farshid; Biancalani, Tommaso; Goldenfeld, Nigel (2017). "Noise-induced symmetry breaking far from equilibrium and the emergence of biological homochirality" (PDF). Physical Review E. 95 (3): 032407. Bibcode:2017PhRvE..95c2407J. doi:10.1103/PhysRevE.95.032407. PMID 28415353.
- ^ Jafarpour, Farshid; Biancalani, Tommaso; Goldenfeld, Nigel (2015). "Noise-induced mechanism for biological homochirality of early life self-replicators". Physical Review Letters. 115 (15): 158101. arXiv:1507.00044. Bibcode:2015PhRvL.115o8101J. doi:10.1103/PhysRevLett.115.158101. PMID 26550754. S2CID 9775791.
- ^ Frank, F.C. (1953). "On spontaneous asymmetric synthesis". Biochimica et Biophysica Acta. 11 (4): 459–463. doi:10.1016/0006-3002(53)90082-1. PMID 13105666.
- ^ Clark, Stuart (July–August 1999). "Polarized Starlight and the Handedness of Life". American Scientist. 87 (4): 336. Bibcode:1999AmSci..87..336C. doi:10.1511/1999.4.336.
- ^ Shibata, Takanori; Morioka, Hiroshi; Hayase, Tadakatsu; et al. (17 January 1996). "Highly Enantioselective Catalytic Asymmetric Automultiplication of Chiral Pyrimidyl Alcohol". Journal of the American Chemical Society. 118 (2): 471–472. doi:10.1021/ja953066g.
- ^ Soai, Kenso; Sato, Itaru; Shibata, Takanori (2001). "Asymmetric autocatalysis and the origin of chiral homogeneity in organic compounds". The Chemical Record. 1 (4): 321–332. doi:10.1002/tcr.1017. PMID 11893072.
- ^ Hazen 2005, p. 184
- ^ Meierhenrich, Uwe (2008). Amino acids and the asymmetry of life caught in the act of formation. Berlin: Springer. pp. 76–79. ISBN 978-3540768869.
- ^ Mullen, Leslie (5 September 2005). "Building Life from Star-Stuff". Astrobiology Magazine. Archived from the original on 14 July 2015.
- ^ "Anticipating an RNA world. Some past speculations on the origin of life: where are they today?" by L. E. Orgel and F. H. C. Crick in FASEB J. (1993) Volume 7 pages 238-239.
- ^ Mautner, M. N. (1997), "“Directed panspermia. 3. Strategies and motivation for seeding star-forming clouds”", J. British Interplanetary Soc. 50: 93
- ^ "علماء الكيمياء يعرضون نظريتهم بشأن نشوء الحياة على الأرض". روسيا اليوم. 2015-03-28. Retrieved 2015-03-31.
General and cited sources
- Altermann, Wladyslaw (2009). "Introduction: A Roadmap to Fata Morgana?". In Seckbach, Joseph; Walsh, Maud (eds.). From Fossils to Astrobiology: Records of Life on Earth and the Search for Extraterrestrial Biosignatures. Cellular Origin, Life in Extreme Habitats and Astrobiology. Vol. 12. Dordrecht, the Netherlands; London: Springer. ISBN 978-1-4020-8836-0.
- Bada, Jeffrey L.; Lazcano, Antonio (2009). "The Origin of Life". In Ruse, Michael; Travis, Joseph (eds.). Evolution: The First Four Billion Years. Foreword by Edward O. Wilson. Cambridge: Belknap Press of Harvard University Press. ISBN 978-0-674-03175-3. OCLC 225874308.
- Barton, Nicholas H.; Briggs, Derek E.G.; Eisen, Jonathan A.; et al. (2007). Evolution. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. ISBN 978-0-87969-684-9. OCLC 86090399.
- Bastian, H. Charlton (1871). The Modes of Origin of Lowest Organisms. London; New York: Macmillan and Company. OCLC 42959303. Retrieved 2015-06-06.
- Bernal, J. D. (1951). The Physical Basis of Life. London: Routledge & Kegan Paul.
- Bernal, J. D. (1960). "The Problem of Stages in Biopoesis". In Florkin, M. (ed.). Aspects of the Origin of Life. International Series of Monographs on Pure and Applied Biology. Oxford, UK; New York: Pergamon Press. ISBN 978-1-4831-3587-8.
- Bernal, J. D. (1967) [Reprinted work by A.I. Oparin originally published 1924; Moscow: The Moscow Worker]. The Origin of Life. The Weidenfeld and Nicolson Natural History. Translation of Oparin by Ann Synge. London: Weidenfeld & Nicolson.
- Bock, Gregory R.; Goode, Jamie A., eds. (1996). Evolution of Hydrothermal Ecosystems on Earth (and Mars?). Ciba Foundation Symposium. Vol. 202. Chichester, UK; New York: John Wiley & Sons. ISBN 978-0-471-96509-1.
- Bondeson, Jan (1999). The Feejee Mermaid and Other Essays in Natural and Unnatural History. Ithaca, NY: Cornell University Press. ISBN 978-0-8014-3609-3.
- Bryson, Bill (2004). A Short History of Nearly Everything. London: Black Swan. ISBN 978-0-552-99704-1. OCLC 55589795.
- Calvin, Melvin (1969). Chemical Evolution: Molecular Evolution Towards the Origin of Living Systems on the Earth and Elsewhere. Oxford, UK: Clarendon Press. ISBN 978-0-19-855342-7. OCLC 25220.
- Chaichian, Masud; Rojas, Hugo Perez; Tureanu, Anca (2014). "Physics and Life". Basic Concepts in Physics: From the Cosmos to Quarks. Undergraduate Lecture Notes in Physics. Berlin; Heidelberg: Springer Berlin Heidelberg. doi:10.1007/978-3-642-19598-3_12. ISBN 978-3-642-19597-6. OCLC 900189038. S2CID 115247432.
- Chang, Thomas Ming Swi (2007). Artificial Cells: Biotechnology, Nanomedicine, Regenerative Medicine, Blood Substitutes, Bioencapsulation, and Cell/Stem Cell Therapy. Regenerative Medicine, Artificial Cells and Nanomedicine. Vol. 1. Hackensack, New Jersey: World Scientific. ISBN 978-981-270-576-1. OCLC 173522612.
- Dalrymple, G. Brent (2001). "The age of the Earth in the twentieth century: a problem (mostly) solved". In Lewis, C.L.E.; Knell, S.J. (eds.). The Age of the Earth: from 4004 BC to AD 2002. Geological Society Special Publication. Vol. 190. London: Geological Society of London. pp. 205–221. Bibcode:2001GSLSP.190..205D. doi:10.1144/gsl.sp.2001.190.01.14. ISBN 978-1-86239-093-5. OCLC 48570033. S2CID 130092094.
{{cite book}}:|journal=ignored (help) - Darwin, Charles (1887). Darwin, Francis (ed.). The Life and Letters of Charles Darwin, Including an Autobiographical Chapter. Vol. 3 (3rd ed.). London: John Murray. OCLC 834491774.
- Davies, Paul (1999). The Fifth Miracle: The Search for the Origin of Life. London: Penguin Books. ISBN 978-0-14-028226-9.
- Dawkins, Richard (1996). The Blind Watchmaker (Reissue with a new introduction ed.). New York: W.W. Norton & Company. ISBN 978-0-393-31570-7. OCLC 35648431.
- Dobell, Clifford (1960) [Originally published 1932; New York: Harcourt, Brace & Company]. Antony van Leeuwenhoek and His 'Little Animals'. New York: Dover Publications.
- Dyson, Freeman (1999). Origins of Life (Revised ed.). Cambridge, UK; New York: Cambridge University Press. ISBN 978-0-521-62668-2.
- Fesenkov, V. G. (1959). "Some Considerations about the Primaeval State of the Earth". In Oparin, A.I.; et al. (eds.). The Origin of Life on the Earth. I.U.B. Symposium Series. Vol. 1. Edited for the International Union of Biochemistry by Frank Clark and R.L.M. Synge (English-French-German ed.). London; New York: Pergamon Press. ISBN 978-1-4832-2240-0. International Symposium on the Origin of Life on the Earth (held at Moscow, 19–24 August 1957)
- Hazen, Robert M. (2005). Genesis: The Scientific Quest for Life's Origin. Washington, DC: Joseph Henry Press. ISBN 978-0-309-09432-0. OCLC 60321860.
- Huxley, Thomas Henry (1968) [1897]. "VIII Biogenesis and Abiogenesis [1870]". Discourses, Biological and Geological. Collected Essays. Vol. VIII (Reprint ed.). New York: Greenwood Press. OCLC 476737627.
- Klyce, Brig (22 January 2001). "Panspermia Asks New Questions" in The Search for Extraterrestrial Intelligence (SETI) in the Optical Spectrum III. 4273, Bellingham, WA: SPIE. doi:10.1117/12.435366. Proceedings of the SPIE held at San Jose, California, 22–24 January 2001
- Lane, Nick (2009). Life Ascending: The 10 Great Inventions of Evolution (1st American ed.). New York: W.W. Norton & Company. ISBN 978-0-393-06596-1. OCLC 286488326.
- Lankenau, Dirk-Henner (2011). "Two RNA Worlds: Toward the Origin of Replication, Genes, Recombination and Repair". In Egel, Richard; Lankenau, Dirk-Henner; Mulkidjanian, Armen Y. (eds.). Origins of Life: The Primal Self-Organization. Heidelberg: Springer. doi:10.1007/978-3-642-21625-1. ISBN 978-3-642-21624-4. OCLC 733245537.
- Lennox, James G. (2001). Aristotle's Philosophy of Biology: Studies in the Origins of Life Science. Cambridge Studies in Philosophy and Biology. Cambridge, UK; New York: Cambridge University Press. ISBN 978-0-521-65976-5.
- Michod, Richard E. (1999). Darwinian Dynamics: Evolutionary Transitions in Fitness and Individuality. Princeton, NJ: Princeton University Press. ISBN 978-0-691-02699-2. OCLC 38948118.
- Oparin, A.I. (1953) [Originally published 1938; New York: The Macmillan Company]. The Origin of Life. Translation and new introduction by Sergius Morgulis (2nd ed.). Mineola, NY: Dover Publications. ISBN 978-0-486-49522-4.
- Raven, Peter H.; Johnson, George B. (2002). Biology (6th ed.). Boston: McGraw-Hill. ISBN 978-0-07-112261-0. OCLC 45806501.
- Ross, Alexander (1652). Arcana Microcosmi. Vol. II. London. OCLC 614453394.
- Sheldon, Robert B. (22 September 2005). "Astrobiology and Planetary Missions" in Astrobiology and Planetary Missions. 5906: 59061I, Bellingham, WA: SPIE. doi:10.1117/12.663480. Proceedings of the SPIE held at San Diego, California, 31 July–2 August 2005
- Tyndall, John (1905) [Originally published 1871; London; New York: Longmans, Green & Co.; D. Appleton and Company]. Fragments of Science. Vol. 2 (6th ed.). New York: P.F. Collier & Sons. OCLC 726998155.
- Voet, Donald; Voet, Judith G. (2004). Biochemistry. Vol. 1 (3rd ed.). New York: John Wiley & Sons. ISBN 978-0-471-19350-0.
- Yarus, Michael (2010). Life from an RNA World: The Ancestor Within. Cambridge, Massachusetts: Harvard University Press. p. 287. ISBN 978-0-674-05075-4.
وصلات خارجية
| مراجع مكتبية عن أصل الحياة |
- Making headway with the mysteries of life’s origins – Adam Mann (PNAS; 14 April 2021)
- How life began on Earth – Marcia Malory (Earth Facts; 2015)
- The Origins of Life – Richard Dawkins et al. (BBC Radio; 2004)
- Life in the Universe – Essay by Stephen Hawking (1996)
- CS1 errors: periodical ignored
- CS1 maint: unflagged free DOI
- مقالات بالمعرفة بحاجة لذكر رقم الصفحة بالمصدر from June 2014
- Short description is different from Wikidata
- Articles with hatnote templates targeting a nonexistent page
- Pages using columns-list with unknown parameters
- أصل الحياة
- تطور
- أيض
- علم الأحياء التطوري
- ظواهر حيوية هامة نشوئياً
- Astrobiology
- Beginnings
- Evolutionarily significant biological phenomena
- Evolutionary biology
- Global events
- Natural events
- Origins
- Prebiotic chemistry



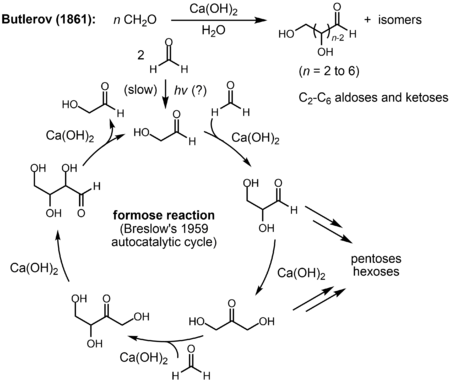
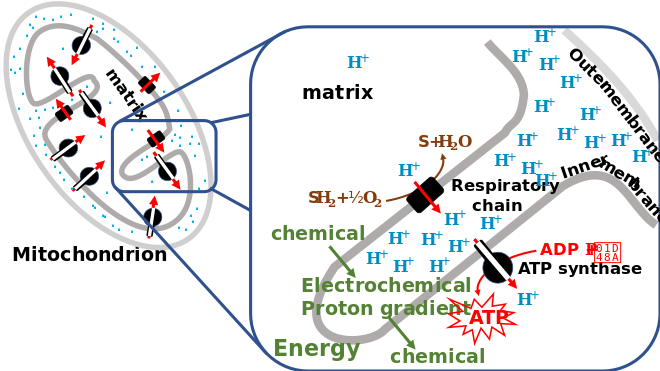
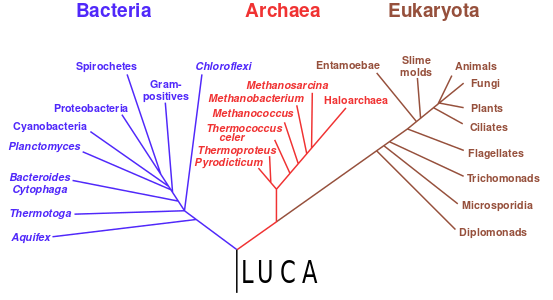
![LUCA systems and environment[11]](/w/images/thumb/e/ed/LUCA_systems_and_environment.svg/622px-LUCA_systems_and_environment.svg.png)

